Product Overview
eRegulatory
Cloudbyz eRegulatory solution offers a cloud-based repository forof all your clinical trial documents. Digitally capture, manage, share, and store all clinical trial-related documents with a centralized overview. Manage essential trial documents, stay inspection ready, and enable real-time visibility for CROs, sponsors, monitors, and other stakeholders in a clinical trial.
With Cloudbyz eRegulatory, research sites can streamline regulatory workflows with paperless binders and an electronic delegation log.
Specifications
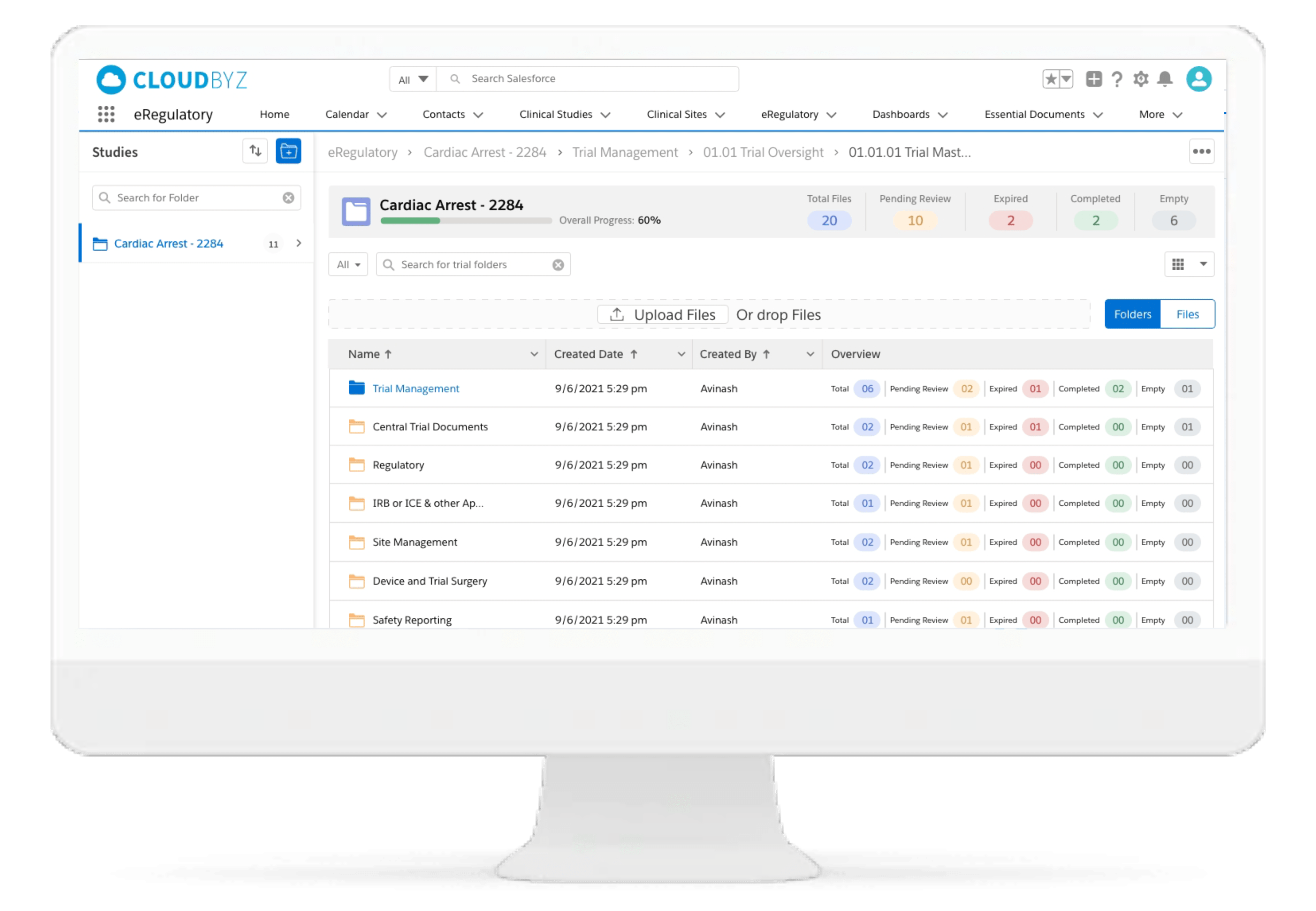
Centralized File Management
Cloudbyz eRegulatory solution enables sites to manage common files such as CVs, Medical Licenses, Credentialing certifications, training logs, etc in a centralized location and automatically align common documents to study as soon as study setup is complete.
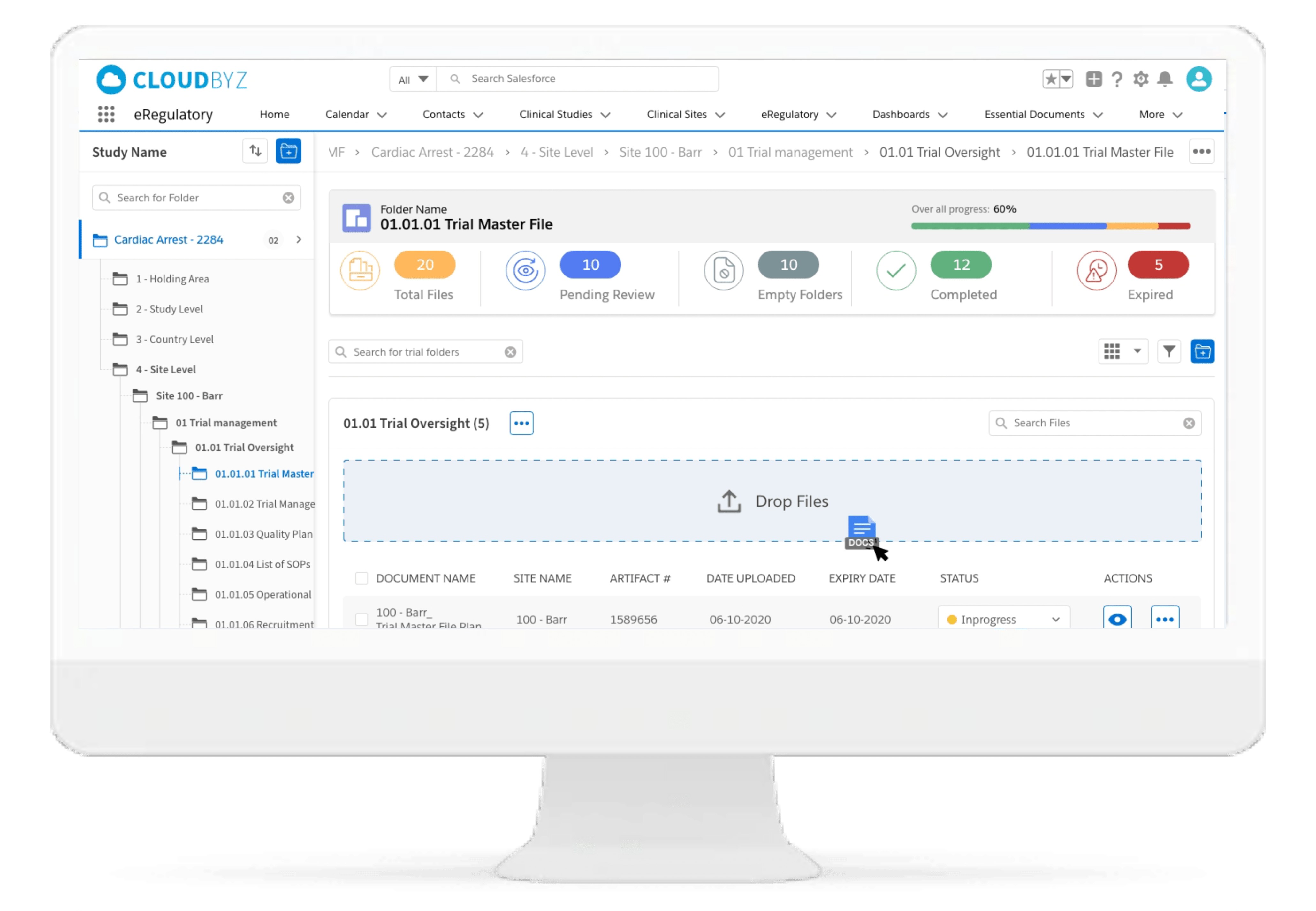
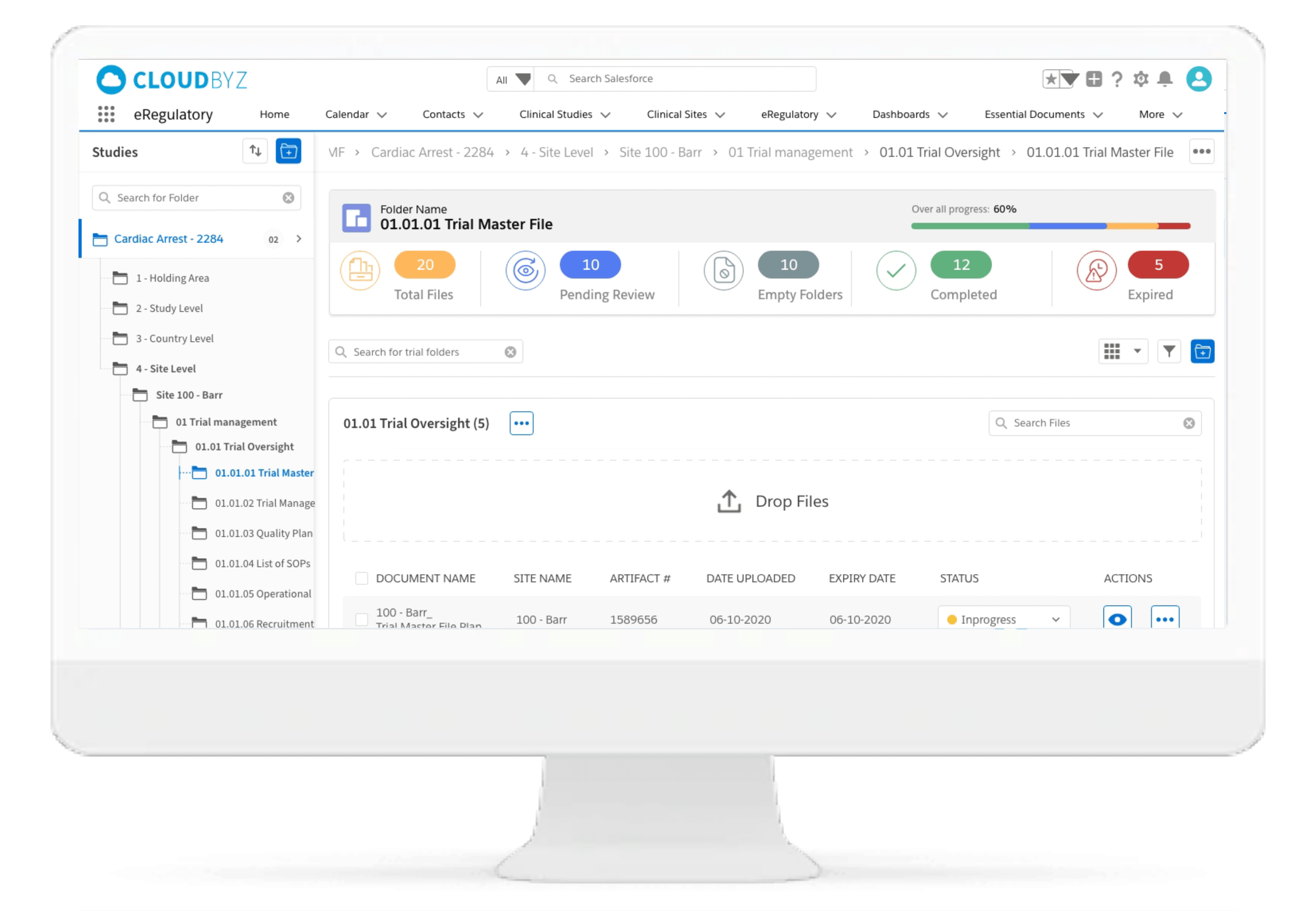
Drag & Drop Upload
The drag & drop functionality in the eRegulatory ensures flexibility with easy movement of one or more files for uploading in the desired electronic trial master file folder.
Generate Document
It facilitates easy document generation in CTMS such as site green light process, protocol deviation, adverse events and trip reports and save to eRegulatory by click of button.
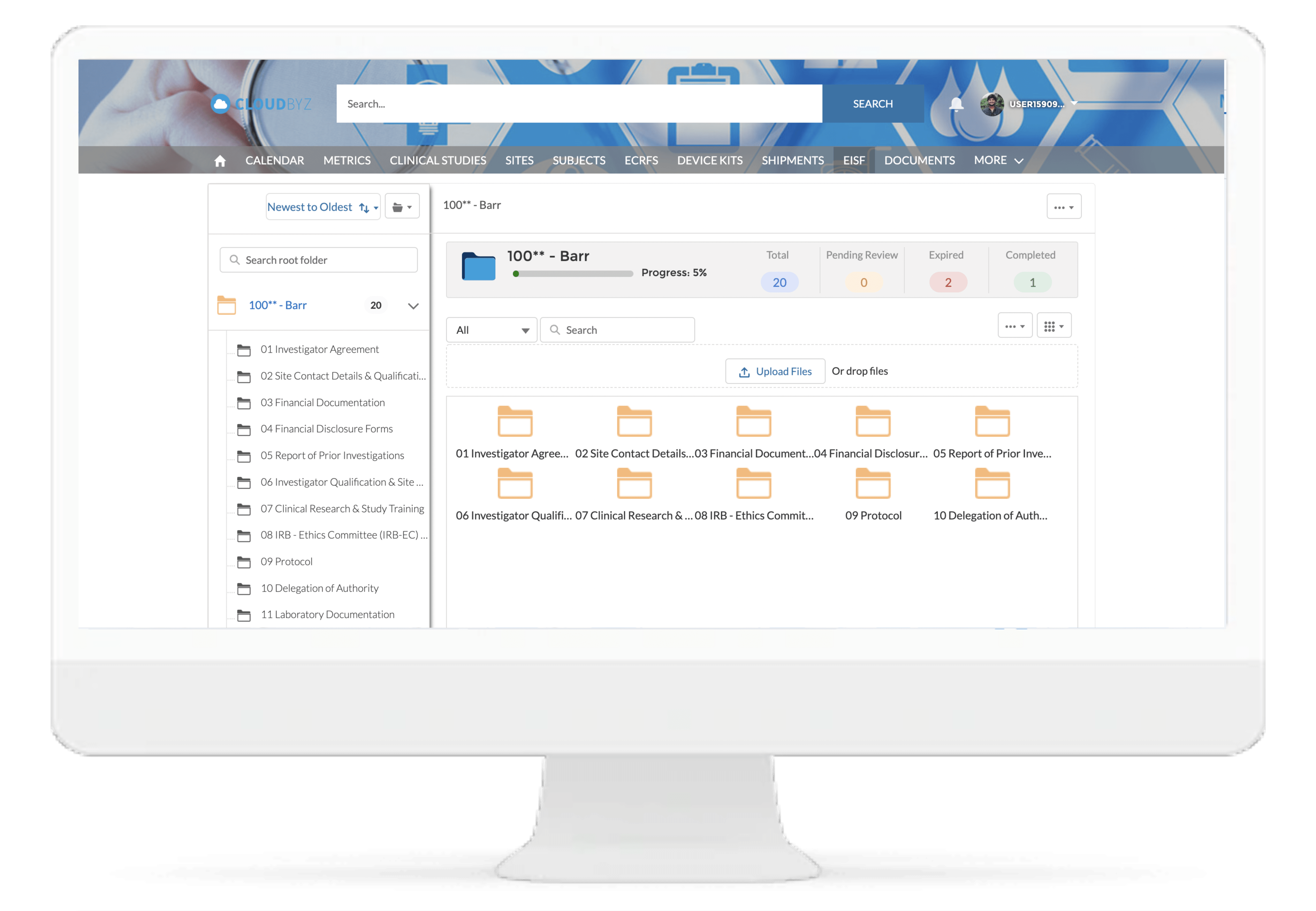
Digital Site Binder Management
Cloudbyz eRegulatory solution comes with template-based and configurable site binder structures. The solution supports the DIA Reference Model for eISF in addition to custom folder structure based on the study and client needs.
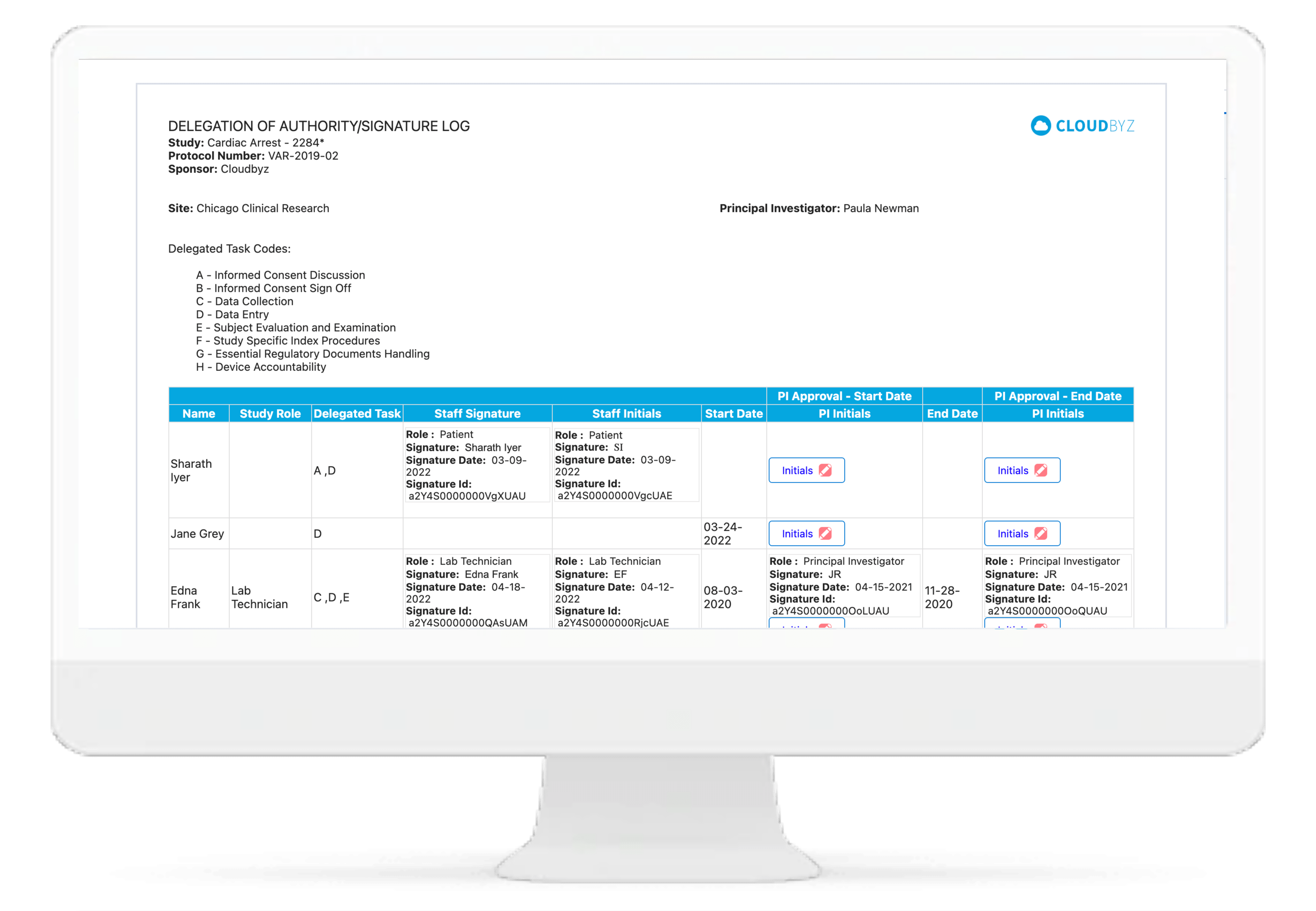
Electronic DOA
Cloudbyz eRegulatory solution comes with automatic generation of electronic delegation of authority log (eDOA) based on the team roles and assigned responsibilities. The study team can sign on the eDOA log through inbuilt electronic signatures. The log can be maintained live for the entire duration of study and can be generated for filing into the site binder and/or the eTMF.
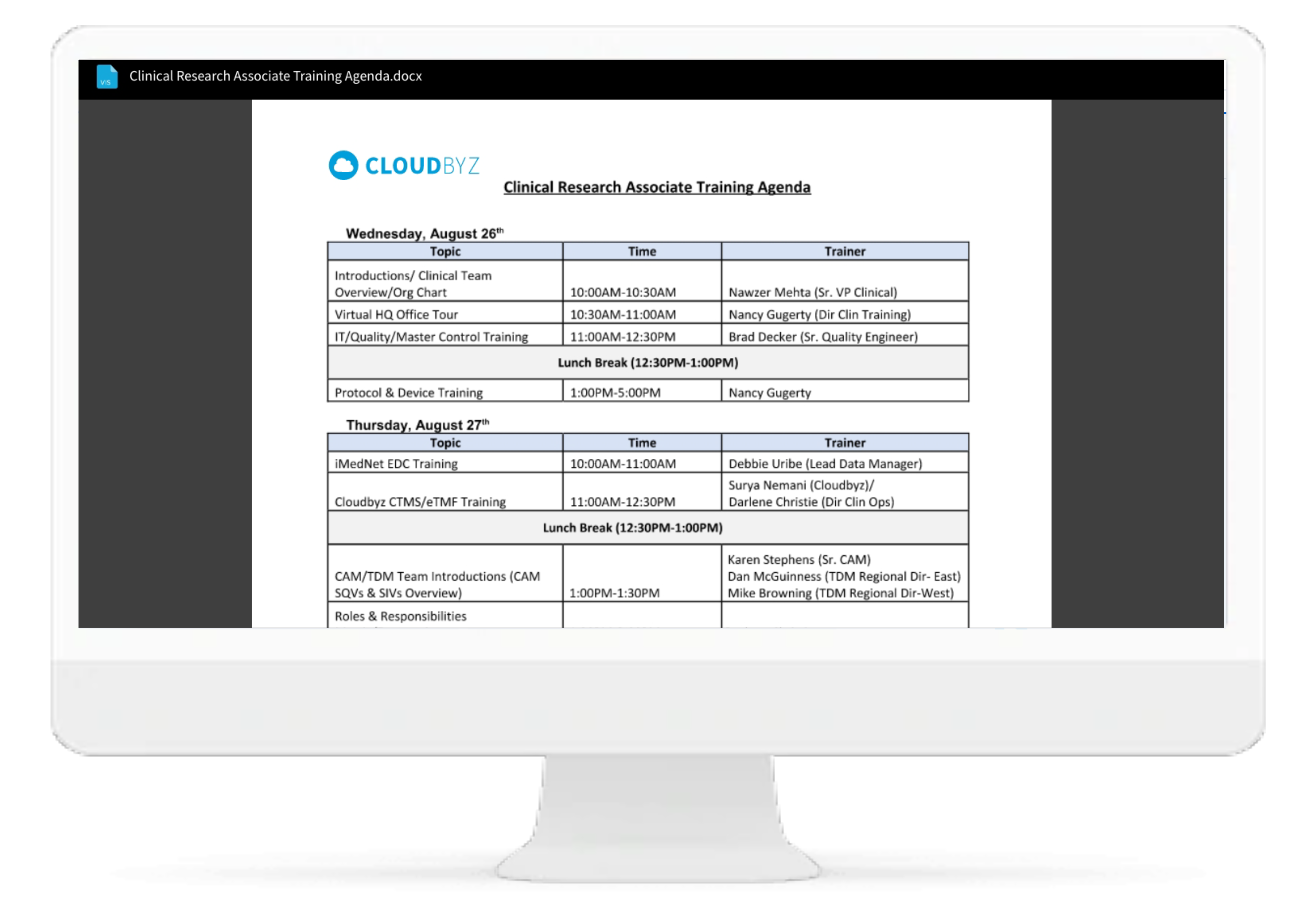
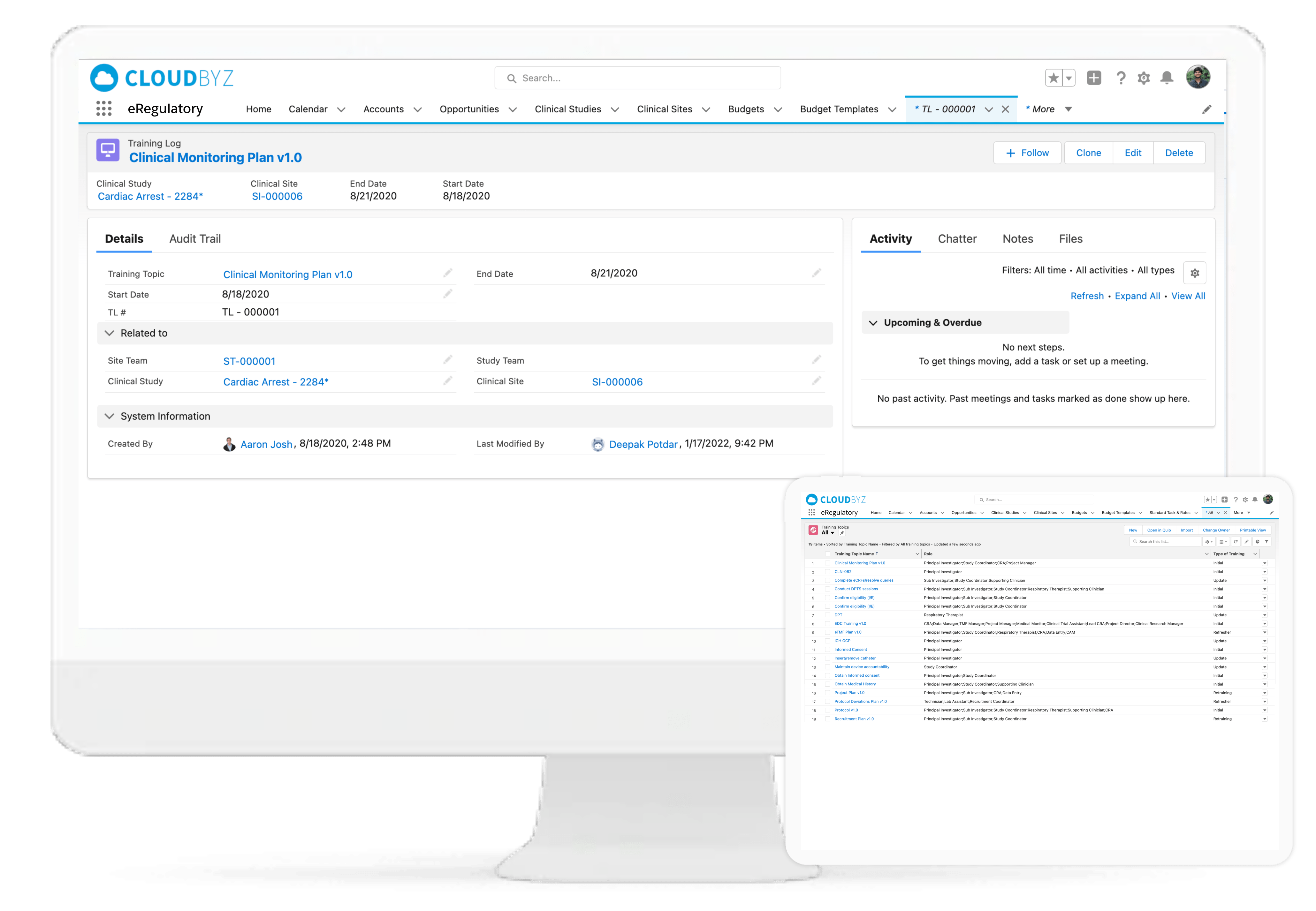
Training Tracker
Cloudbyz eRegulatory tracks training logs for all site staff involved in clinical study. The training log can be routed for electronic Signature and be saved in the regulatory.
Remote Access
Cloudbyz eRegulatory comes with portal capabilities where external stakeholders can login to review real-time updates and access regulatory documents. With permission enabled, Sponsors and CROs can download regulatory documents and save them in their eTMF system.
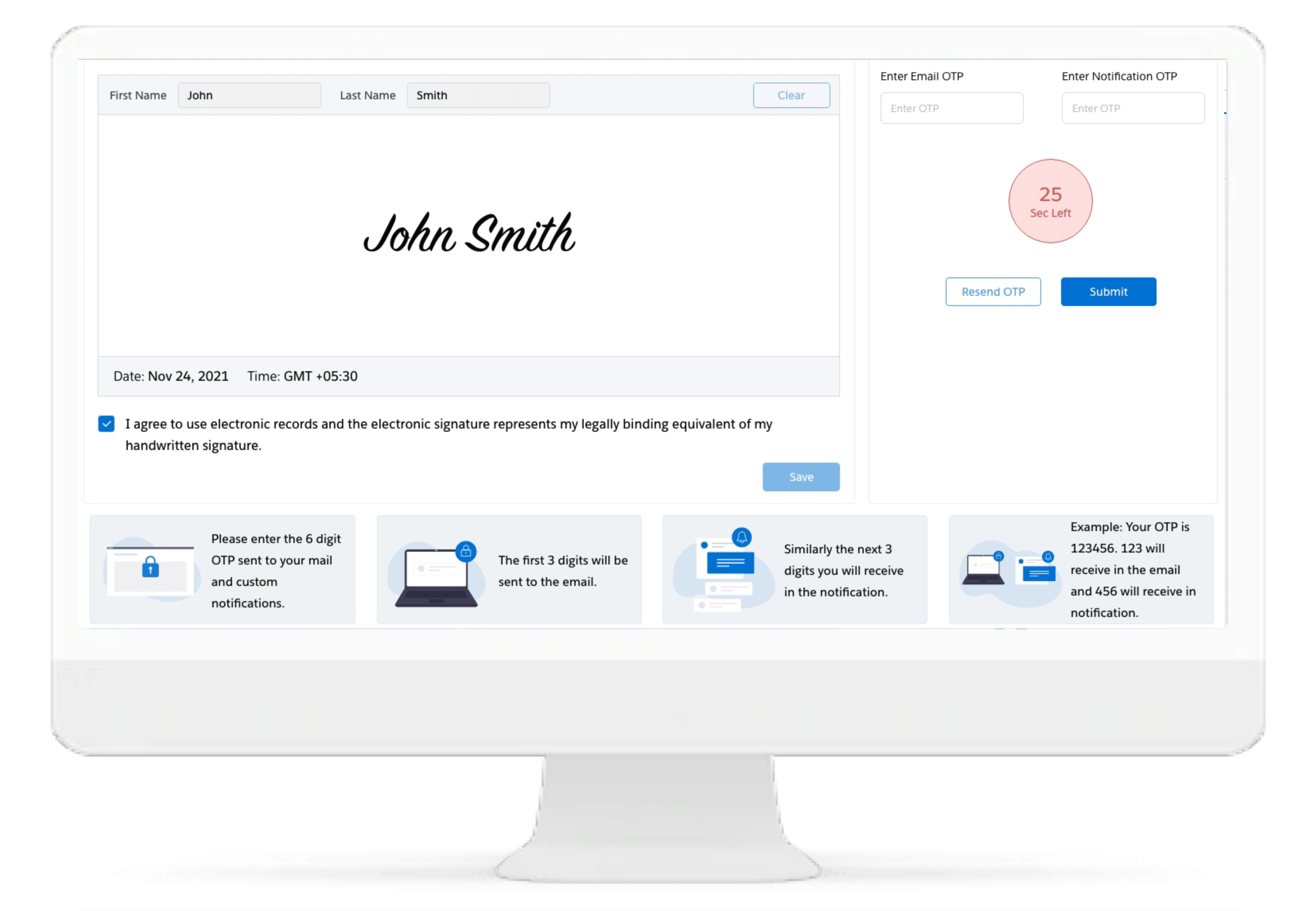
eSignatures
The Cloudbyz eRegulatory solution supports 21 CFR part 11 compliant eSignature capabilities.
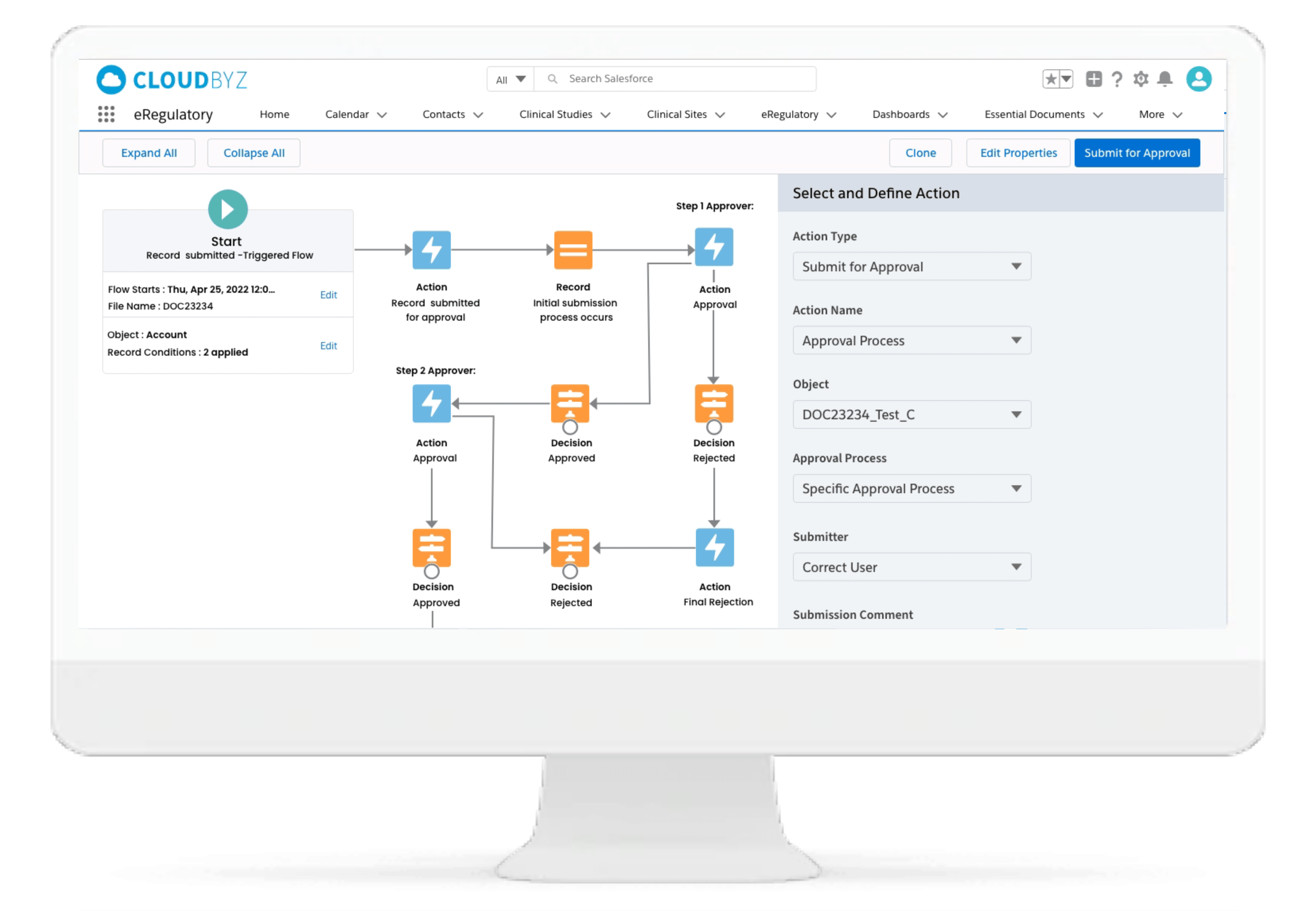
Approvals
Cloudbyz eRegulatory comes with point and click and drag and drop configurable workflow and approval process engine which enables customers to configure their own approval process and workflow automation.
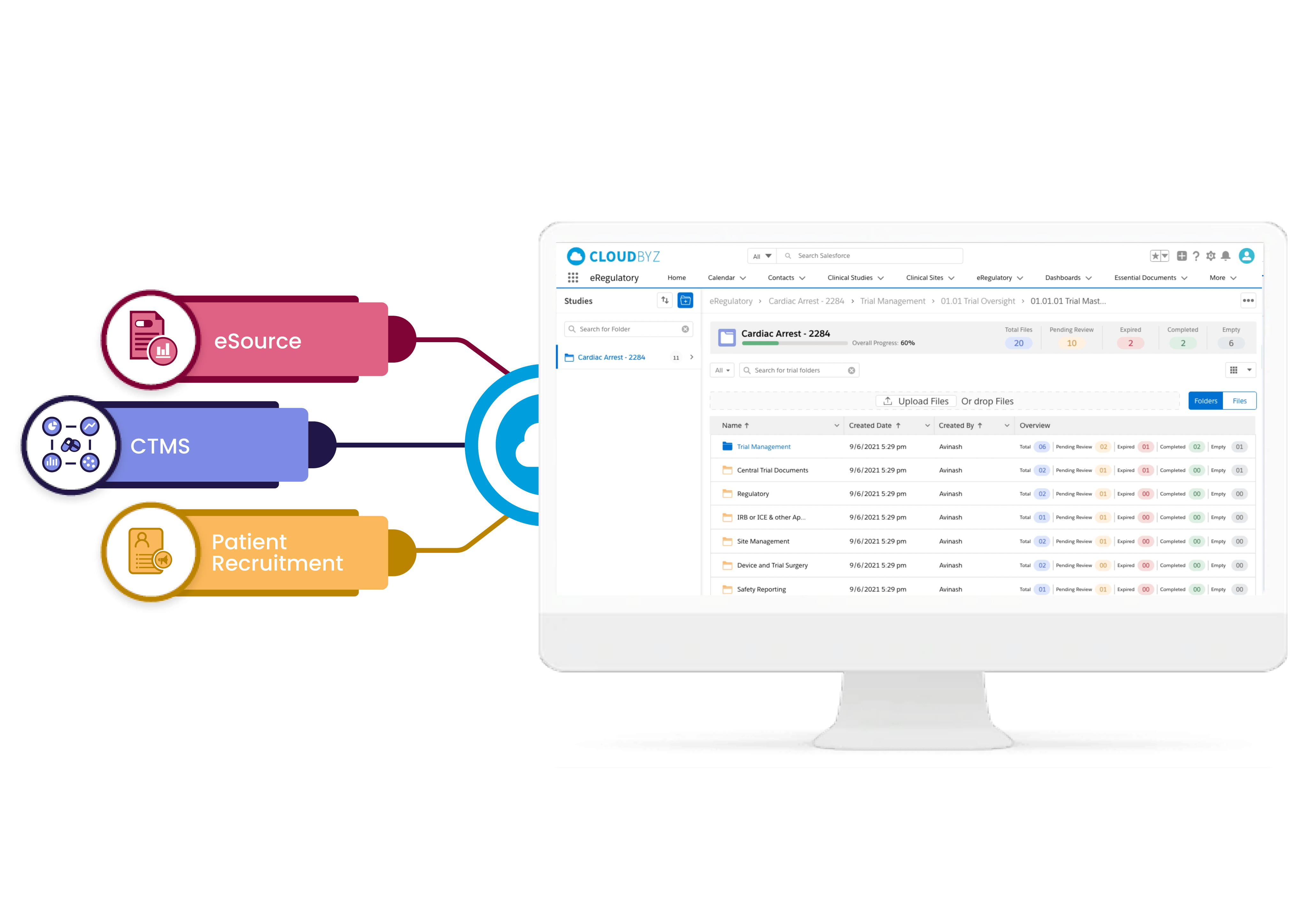
Integrated with eSource
Cloudbyz eRegulatory solution is integrated with eSource, CTMS and Patient Recruitment capabilities and enables seamless collaboration across teams and helps to achieve efficiency.