Product Overview
Randomization & Trial Supply Management
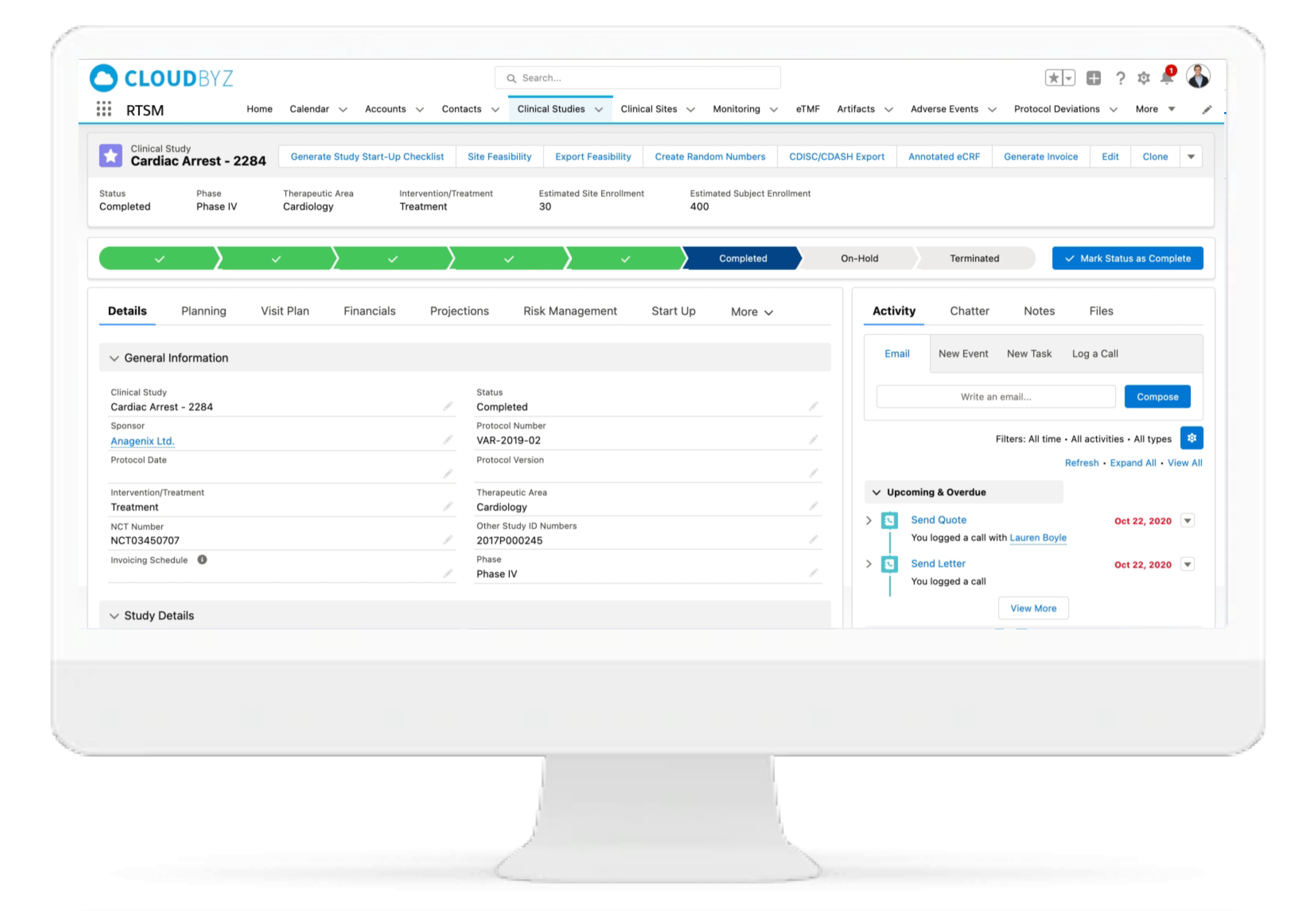
Cloudbyz Randomization & Trial Supply Management (RTSM) solution is completely customizable per the requirements of a clinical study. Our system uses an integrated IWRS (Interactive Web Response System) and effortlessly handles simple to complex randomization schemes with provision for stratifications and multi arm studies. The system performs all the tasks related to trial supply management such as Investigational Product Accountability, centralized inventory tracking, threshold management, automated orders, and notifications.
Specifications
Study Build
With Cloudbyz RTSM solution, set up and visualize studies according to protocol requirements and easily access details such as phase, type, cohorts, therapeutic area, indication, outcomes, eligibility etc. and others.
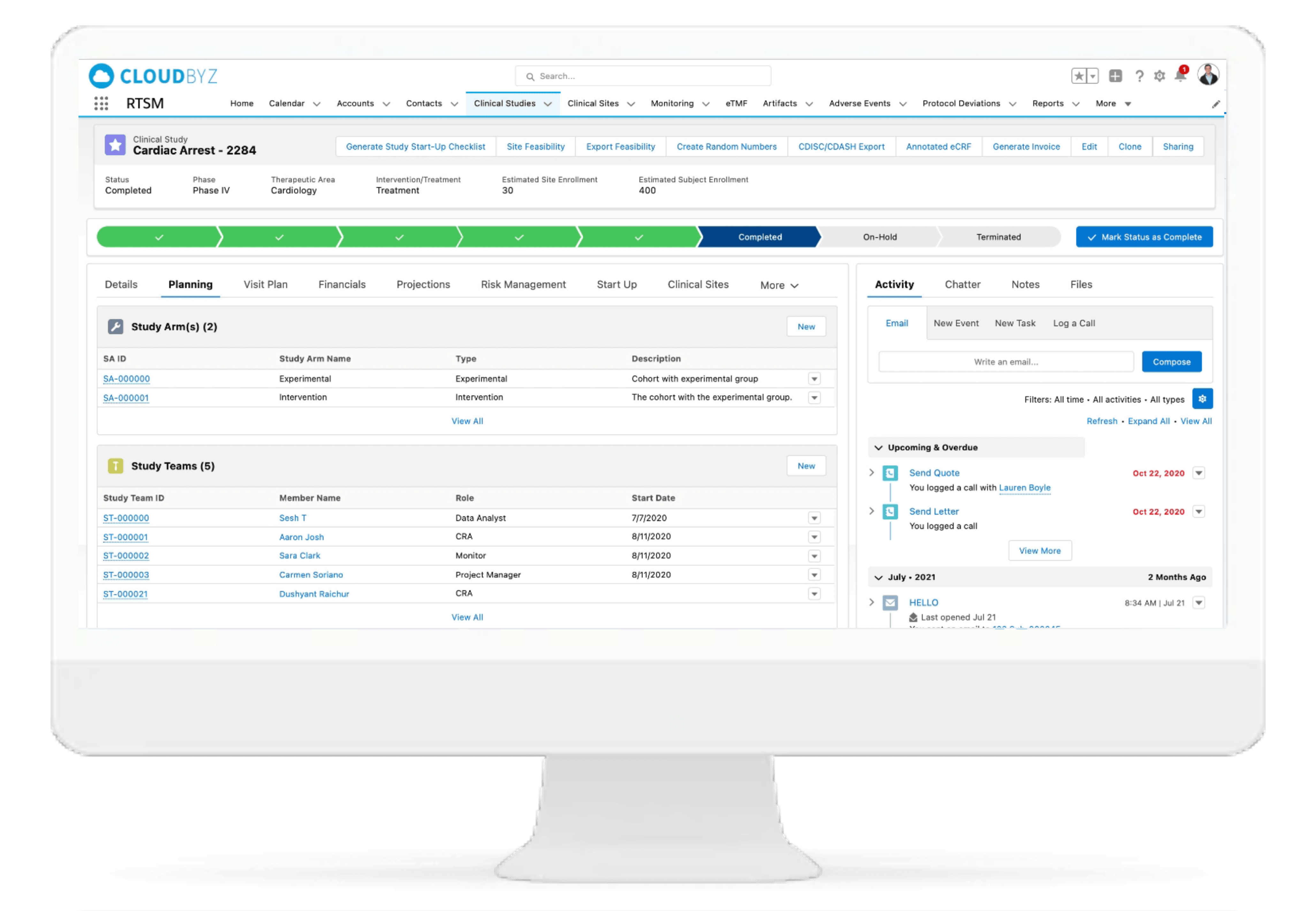
Cohorts
Manage Cohorts easily with fully configurable cohort management features. Open and Close specific cohorts based on enrollment needs with a click of a button.
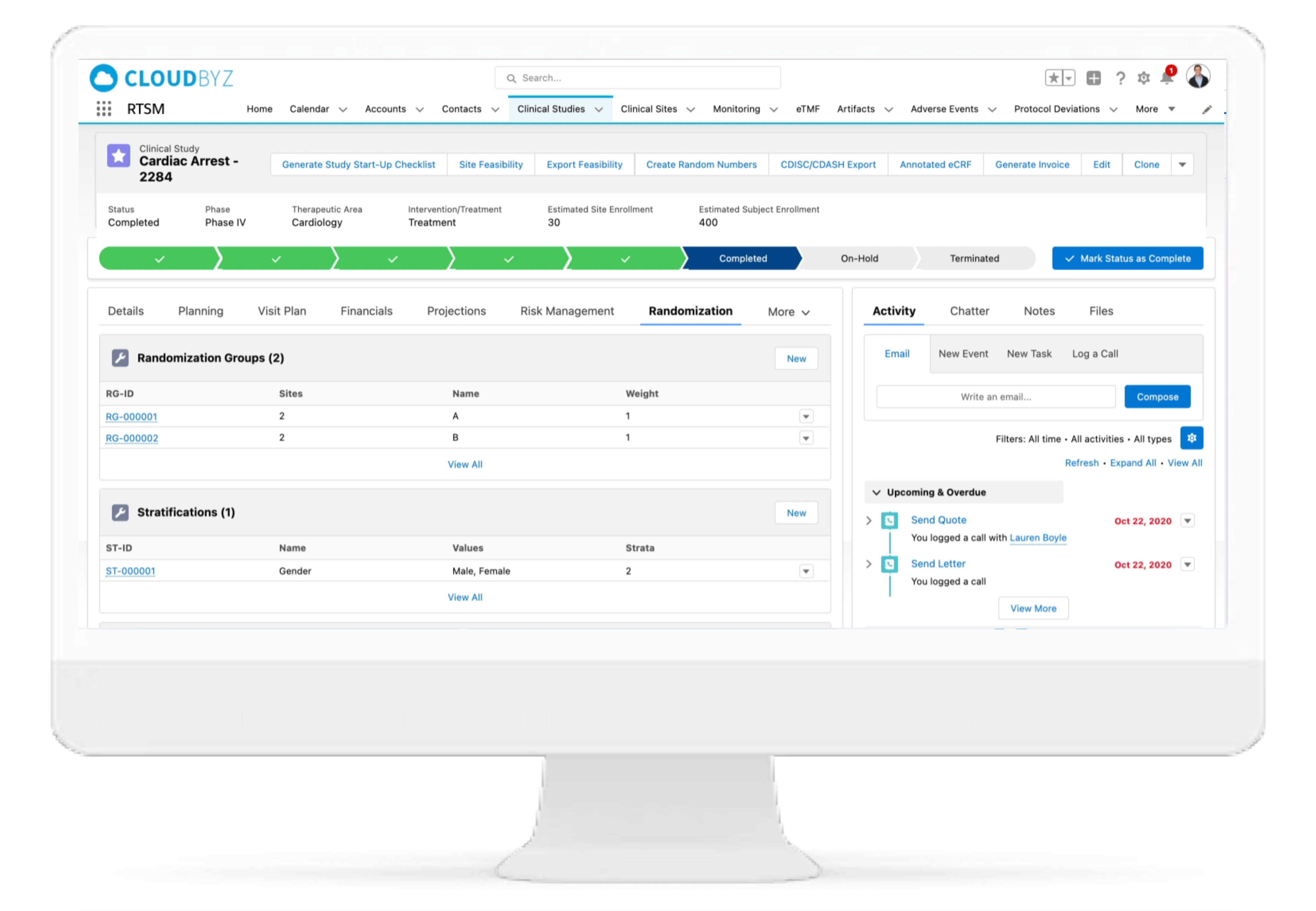
Randomization
Conduct fast and accurate subject randomization based on protocol design. Link all types of randomizations - simple, blocked, and stratified, directly to the site inventory.
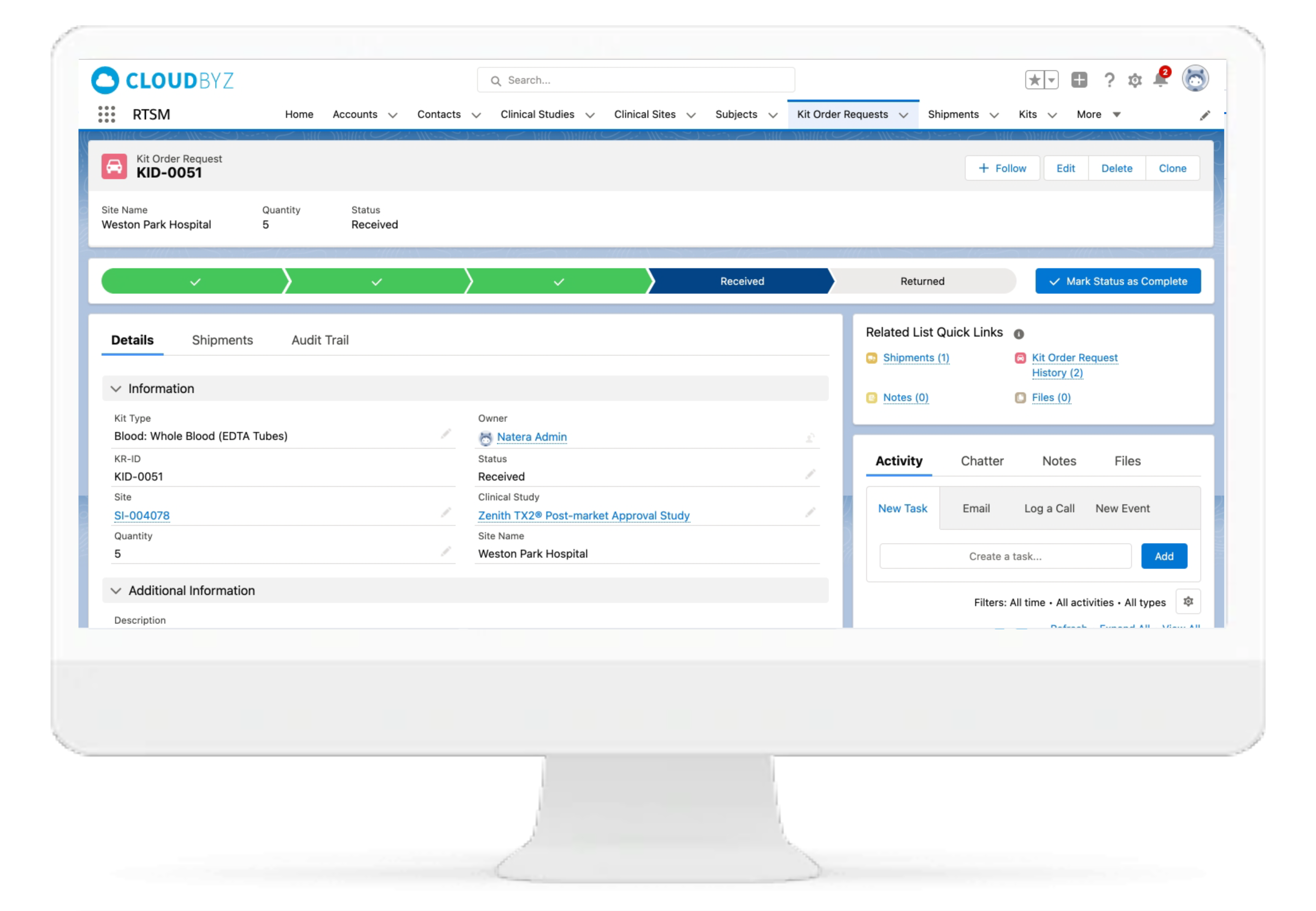
IP Orders Management
The Cloudbyz RTSM system tracks inventory at the site and confirms if a treatment inventory has fallen to a predefined minimum level. System sends an electronic request to the depot for consignment of additional supply to site. The orders can also be placed manually per study team's requirements.
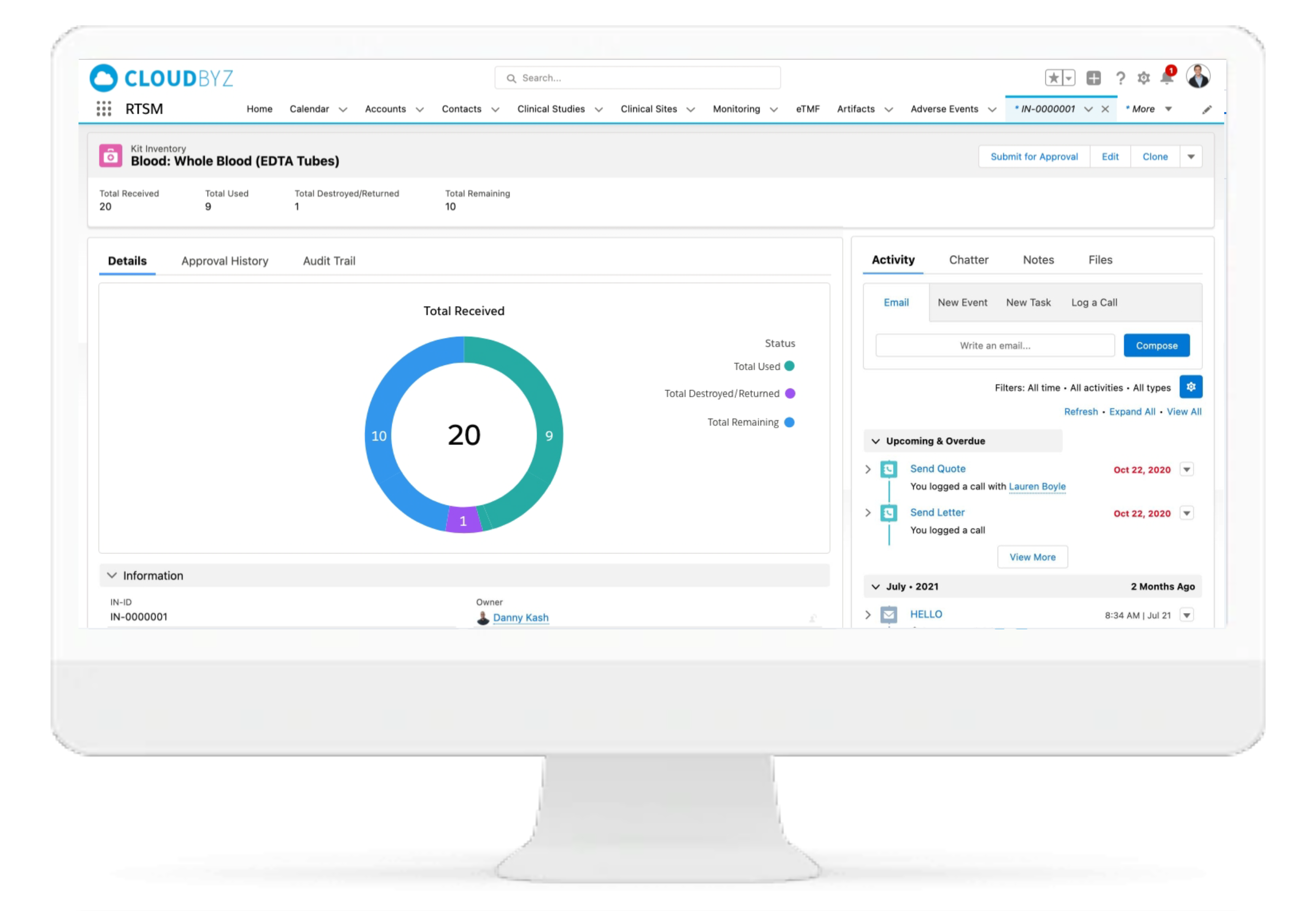
IP Inventory Management
Cloudbyz RTSM solution has automated and centralized inventory tracking and supply management. Features include automated site inventory requests, shipment reminders, quarantine management.
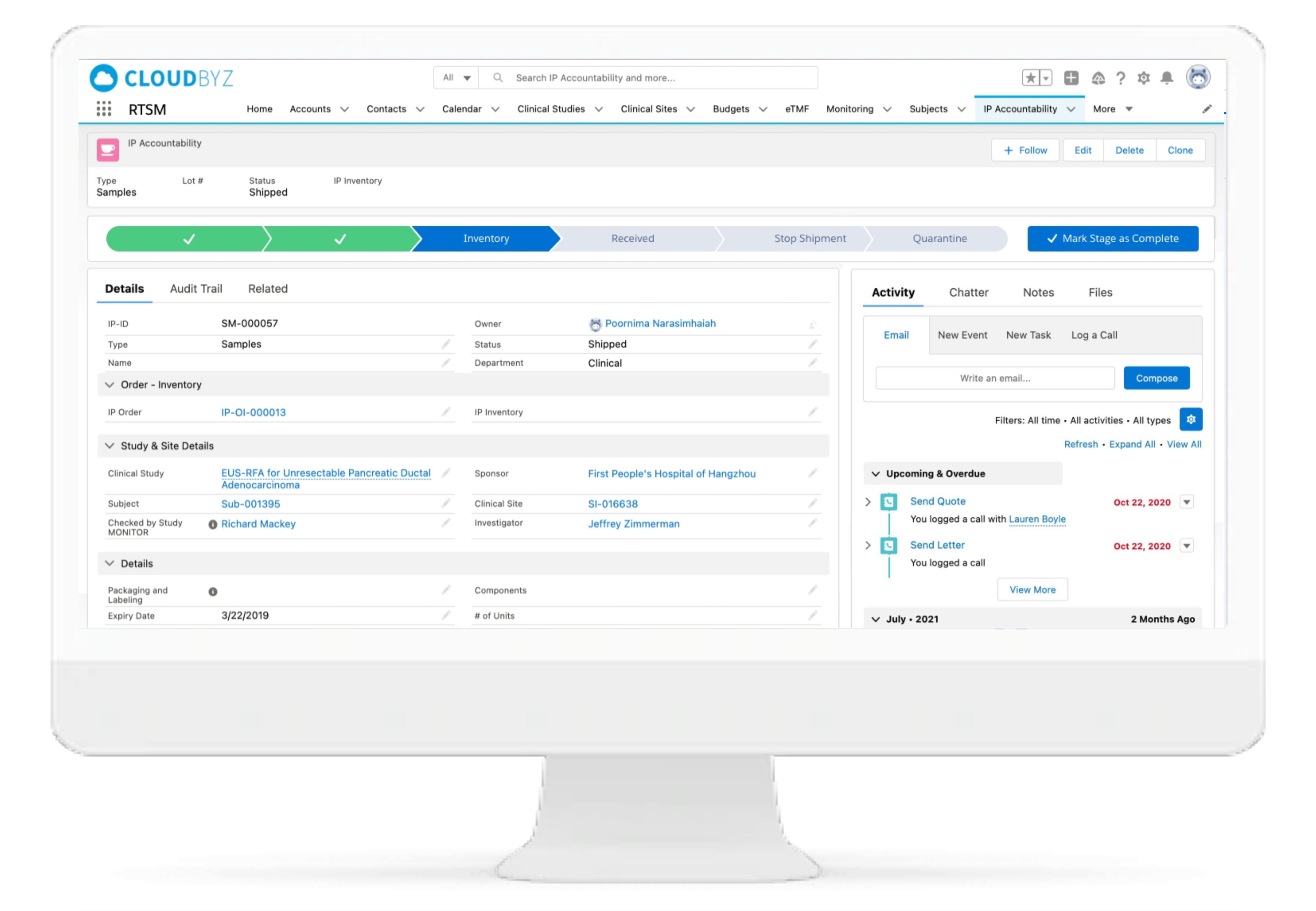
IP Accountability & Reconciliation
With Cloudbyz RTSM solutions, the Investigational Product accountability and reconciliation can be performed centrally from a single location. The solution provides kit and IP status for each site throughout the study thus facilitating superior oversight.
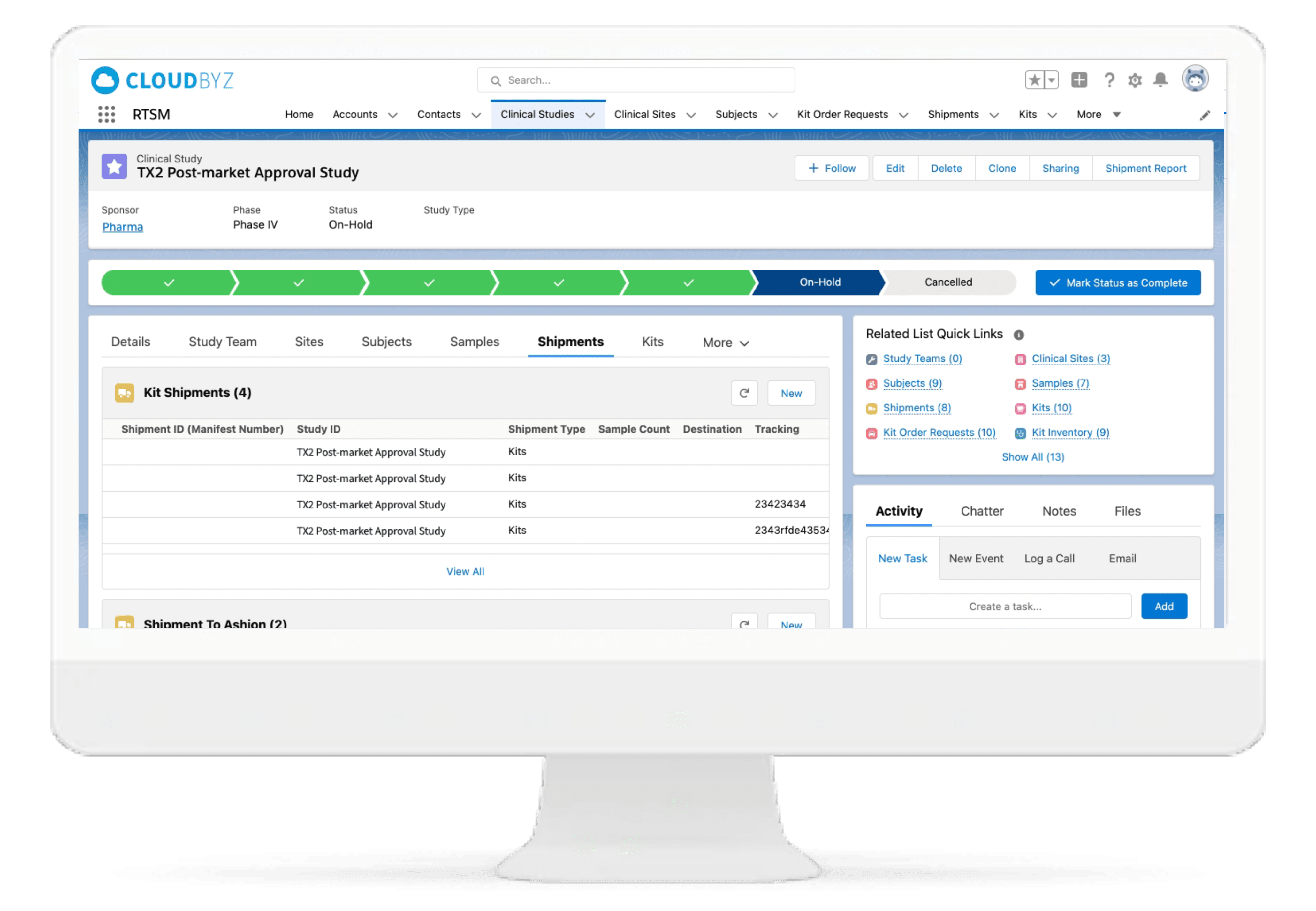
Shipment Management
Track shipments from orders to delivery and returns. Control products from depot to the site, subject, and back. Ensure an uninterrupted drug supply.
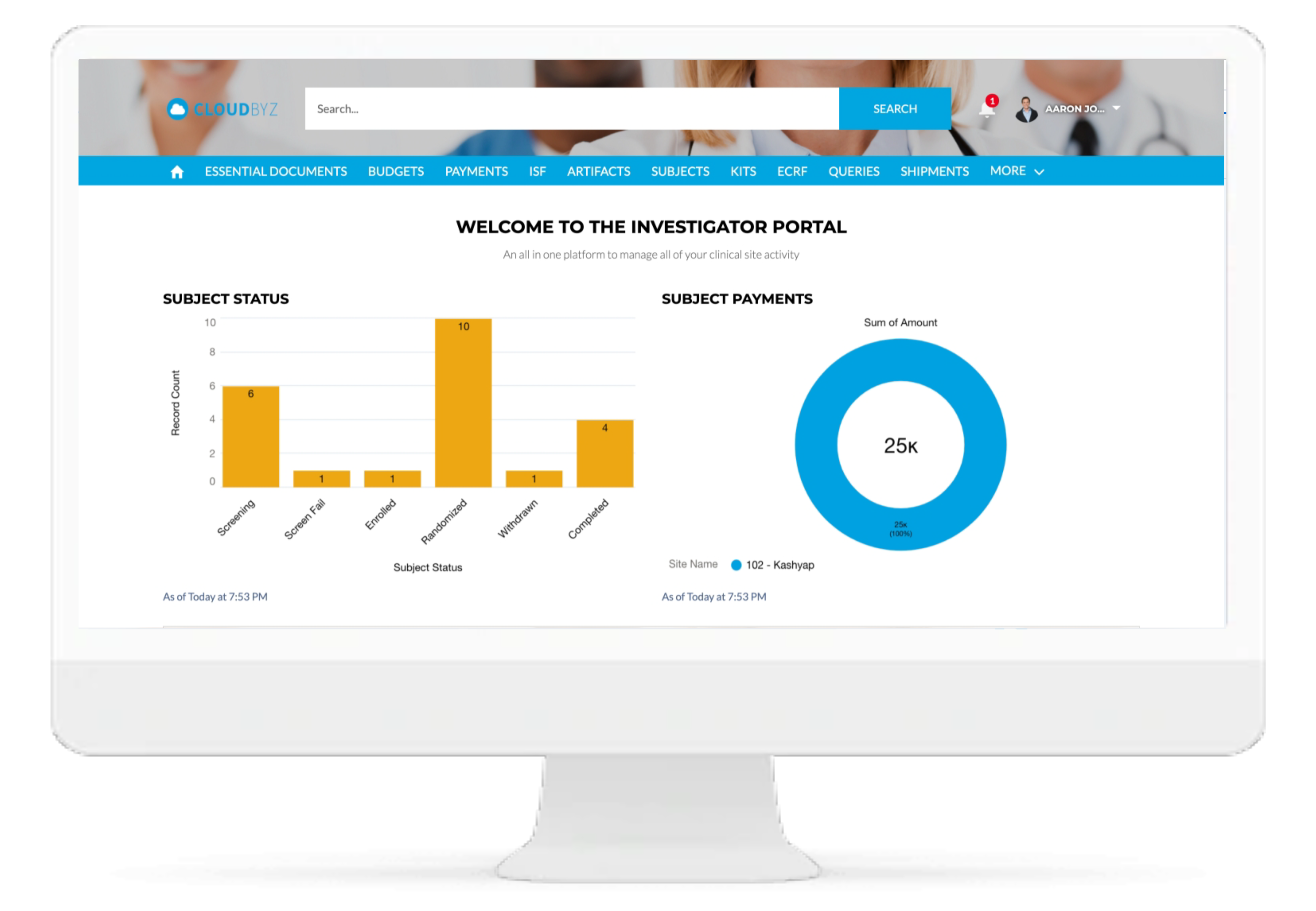
Investigator Portal
Cloudbyz Investigator Portal solution provides a single platform to deliver content and services to clinical research sites. With investigator portal, sites can complete eCRFs, report protocol deviations and adverse events, maintain electronic Investigator Site File, access study information, collect essential documentation, perform investigation product accountability, and communicate with monitors, CROs, Sponsors and other vendors, all from single location.
Patient Portal
The Cloudbyz RTSM solution can be seamlessly integrated to a patient portal that can be utilized for ePRO, eConsent, patient Information sheets, patient Education, eBooks, lab reports and screening results.
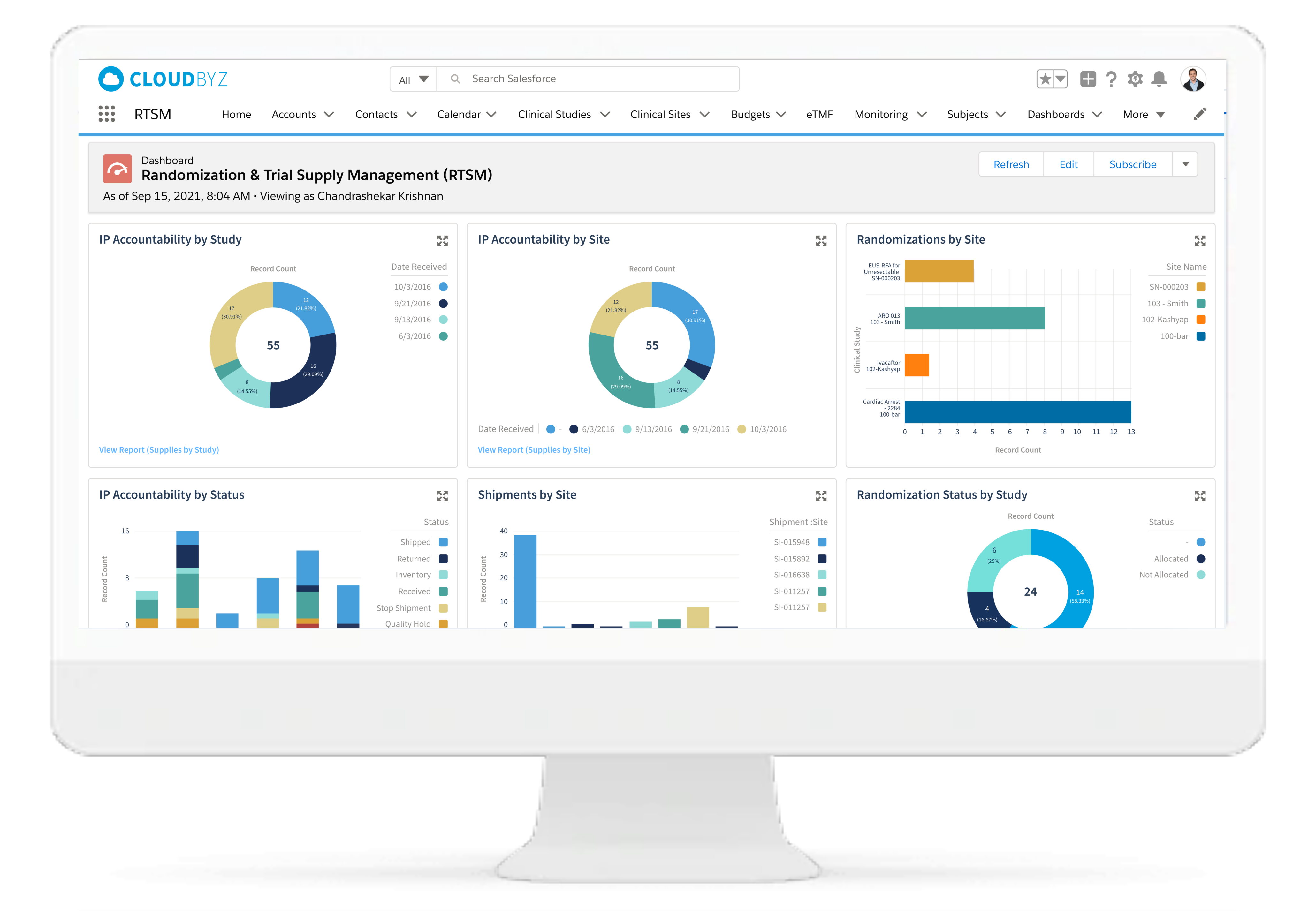
Real-time Analytics with reports and dashboards
The Cloudbyz RTSM solution offers ready to use and customized reports and dashboards to track various metrics. Reports can be created with a point and click configuration to track metrics such as enrollment numbers, Inventory levels, shipment timelines etc.