Product Overview
Risk Based Monitoring
Cloudbyz delivers a Risk Based Monitoring (RBM) Solution that enables sponsors to improve their clinical oversight process, reduce time, costs, effort, and empowers them to review important monitoring activities, in real-time across the sites, all from one place. Our solution has the tools which offer quick results for the sponsors without the need for extensive programming, or even statistical experience.
Specifications
Risk Assessment and Mitigation
Implement and categorize risks into high, medium, and low risks within the system. Prioritize the identified risk based on likelihood, extent and impact these risks might have on the trial integrity. Set up the probable mitigation steps for each category of risk.
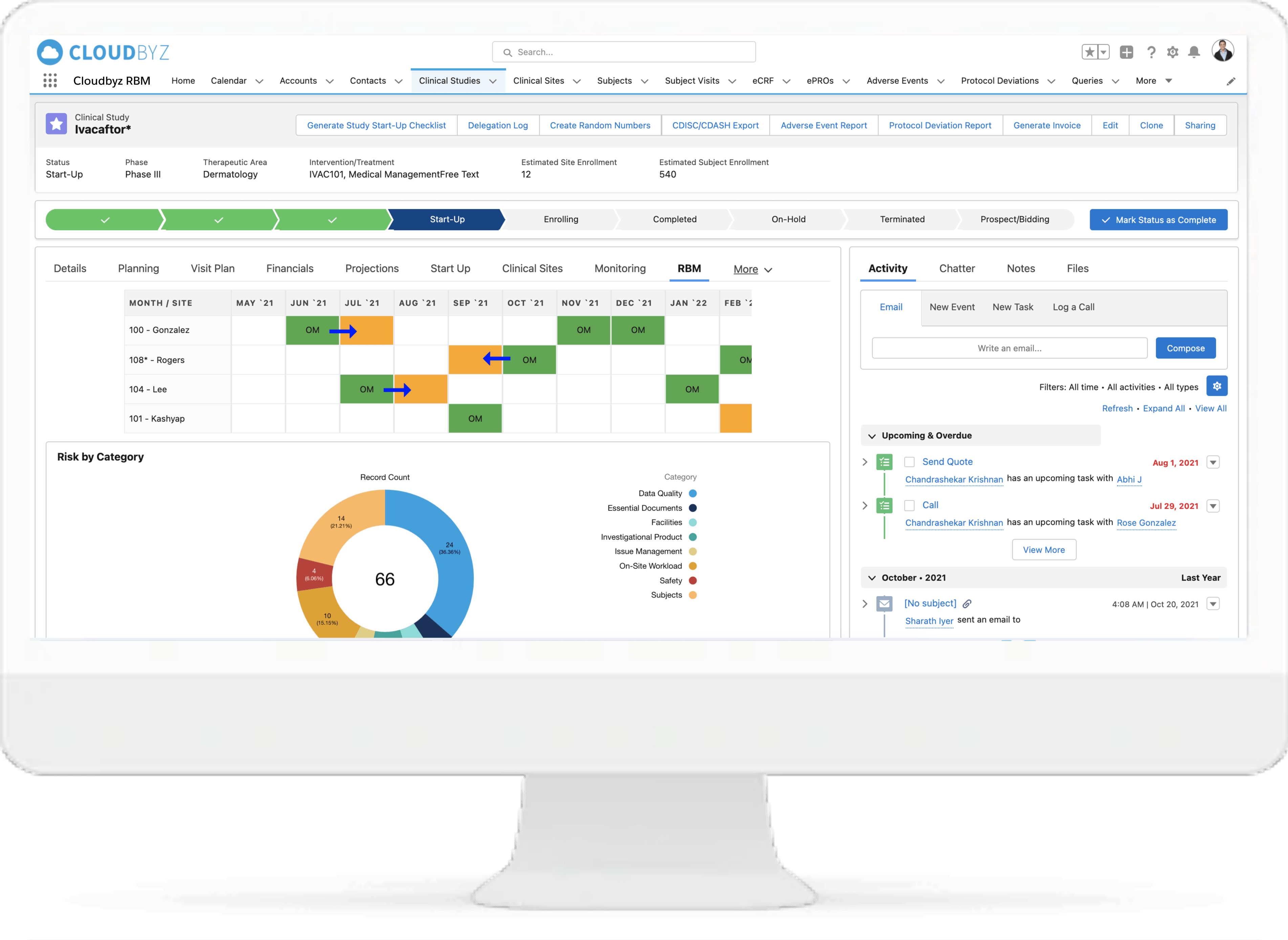
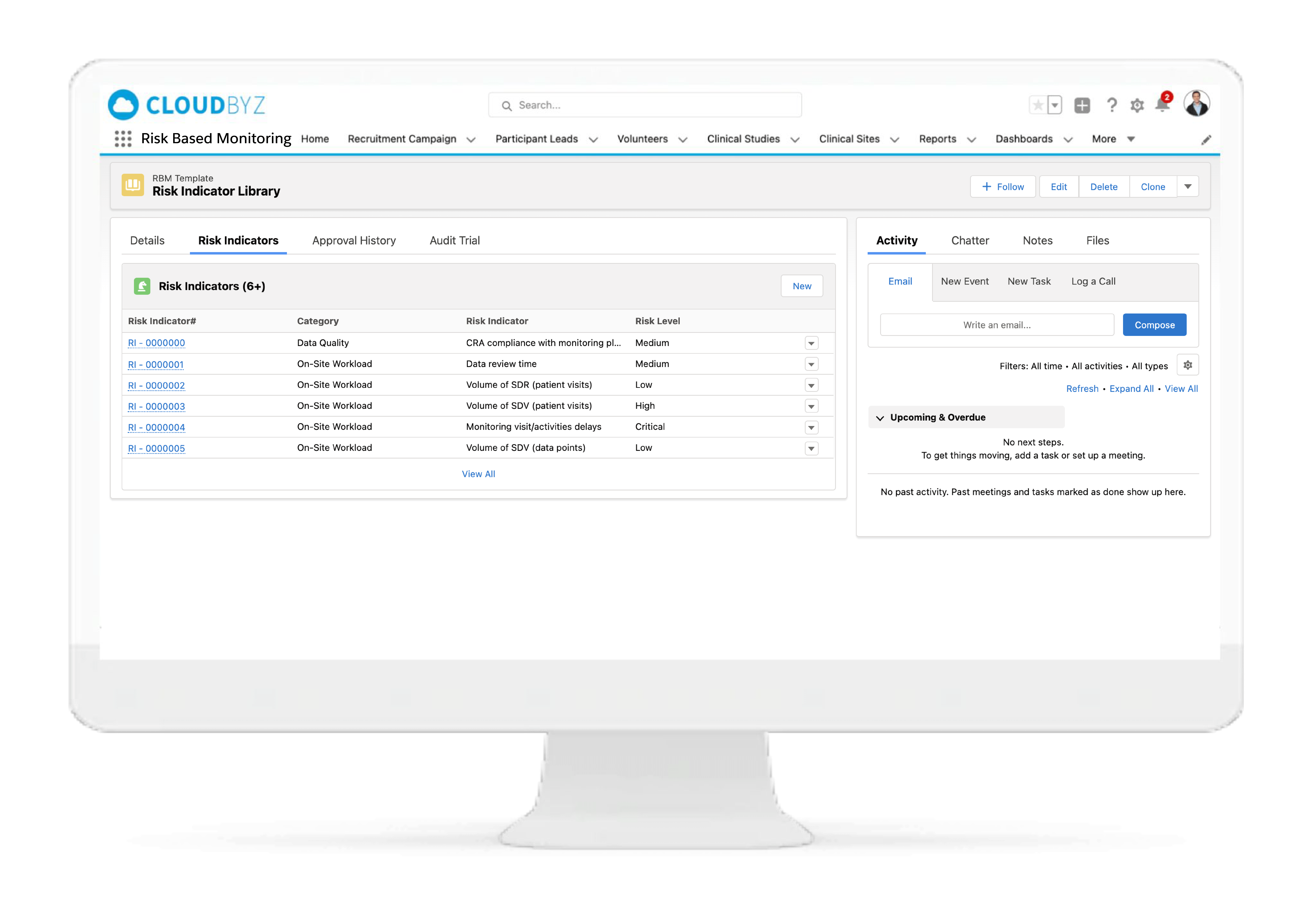
Key Risk Indicators
Identify and apply the most critical data and processes for each type of study. Choose from a collection of Risk Indicators and apply to your study or set up your own. Manage key risk indicators directly within the system to increase data quality for the entire duration of the study
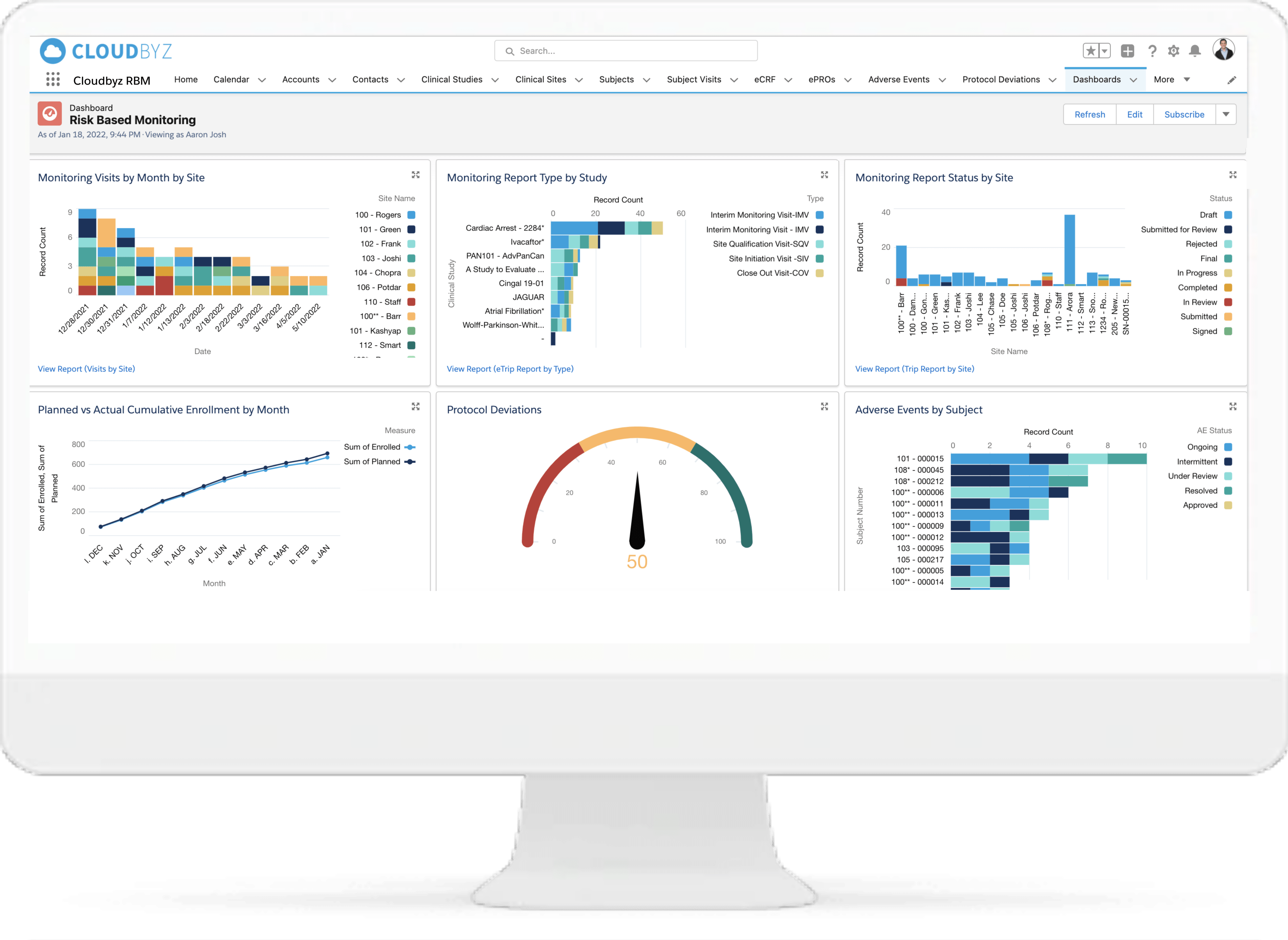
Reports and Dashboards
Select from the available reports and dashboards to view and assess critical metrics. Create custom and ad-hoc reports through just point and click configuration. Subscribe to your favorite or most important reports and receive them in your inbox at your chosen time and date.
Thresholds, Triggers and Notifications
Set up thresholds around the types of data that need to be collected, the exact activities essential to collect these data points, and the range of potential safety and other human subject protection concerns that are inherent to the clinical investigation. Monitors and Project Managers receive these trigger notifications alongwith the suggestion on the recommended mitigation action based on the finalized monitoring plan.
Automations
Use more automated reviews and better analysis with our Risk Based Monitoring solution and reduce the need for manual intervention and on-site monitoring. Schedule monitoring visits with a click of button based on the automatic recommendation.
Integrated
Integrate with various clinical trial Management systems and control your entire process from one location. Extend integrations to multiple systems and automate the implementation of your monitoring plan to the maximum extent.