Product Overview
Safety & Pharmacovigilance
Cloudbyz Safety and Pharmacovigilance (PV) software is a cloud-based solution built natively on the Salesforce platform. It offers 360 degree view across R&D and commercial. It also enables pharma, bio-tech and medical devices companies to make faster and better safety decisions. It also helps to optimize global pharmacovigilance compliance along with easy to integrate risk management feature.
Our pharmacovigilance software solution easily integrates the required data over a centralized cloud-based platform for advanced analytics set-up along with data integrity. It empowers the end-user with proactive pharmacovigilance, smart features with data-backed predictability, scalability and cost-effective support
Specifications
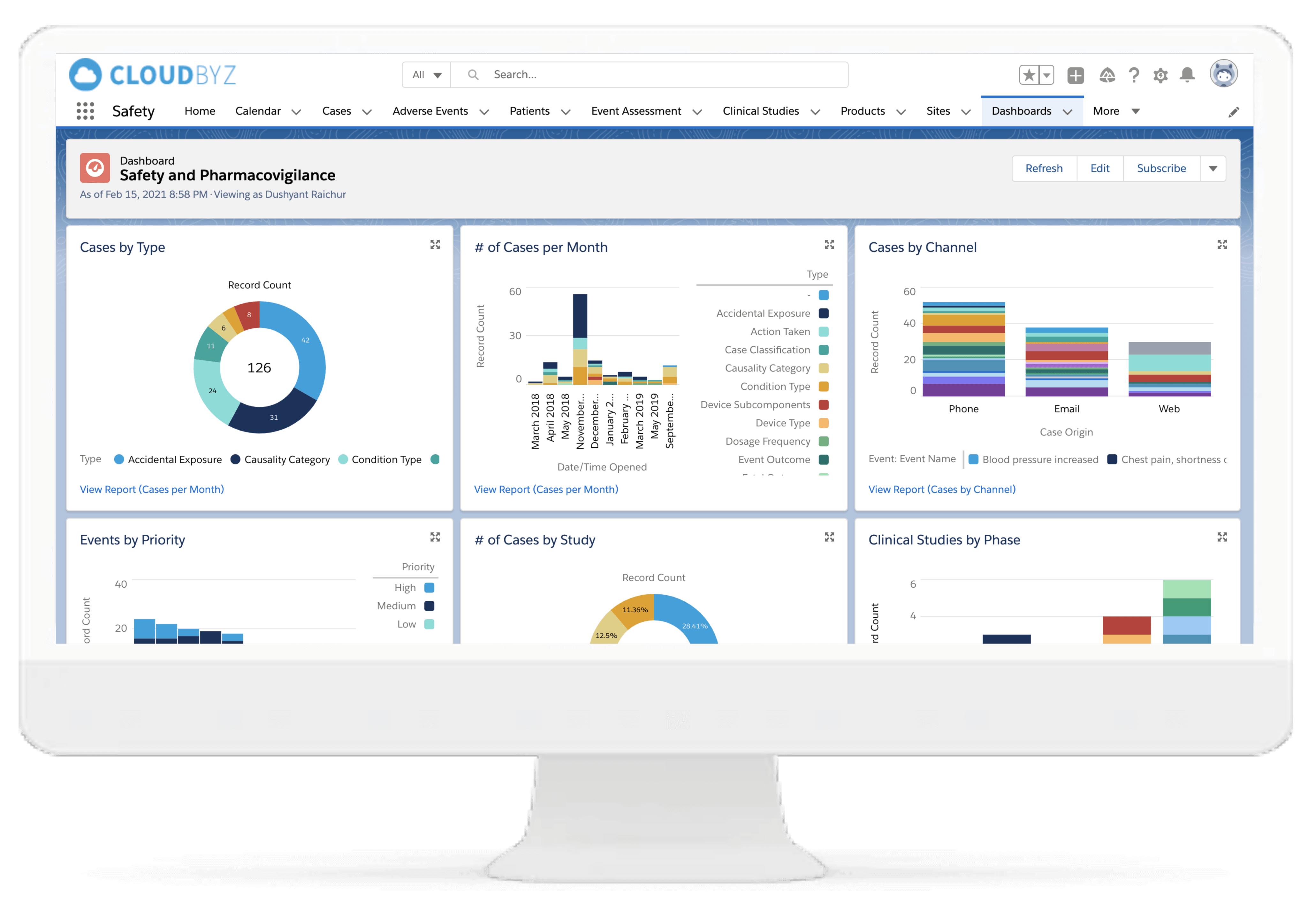
Analytics & Reporting
Facilitates creation of striking, vibrant reports for C-suite executives and end-users to understand insights regarding daily pharmacovigilance operations with bundled reports.
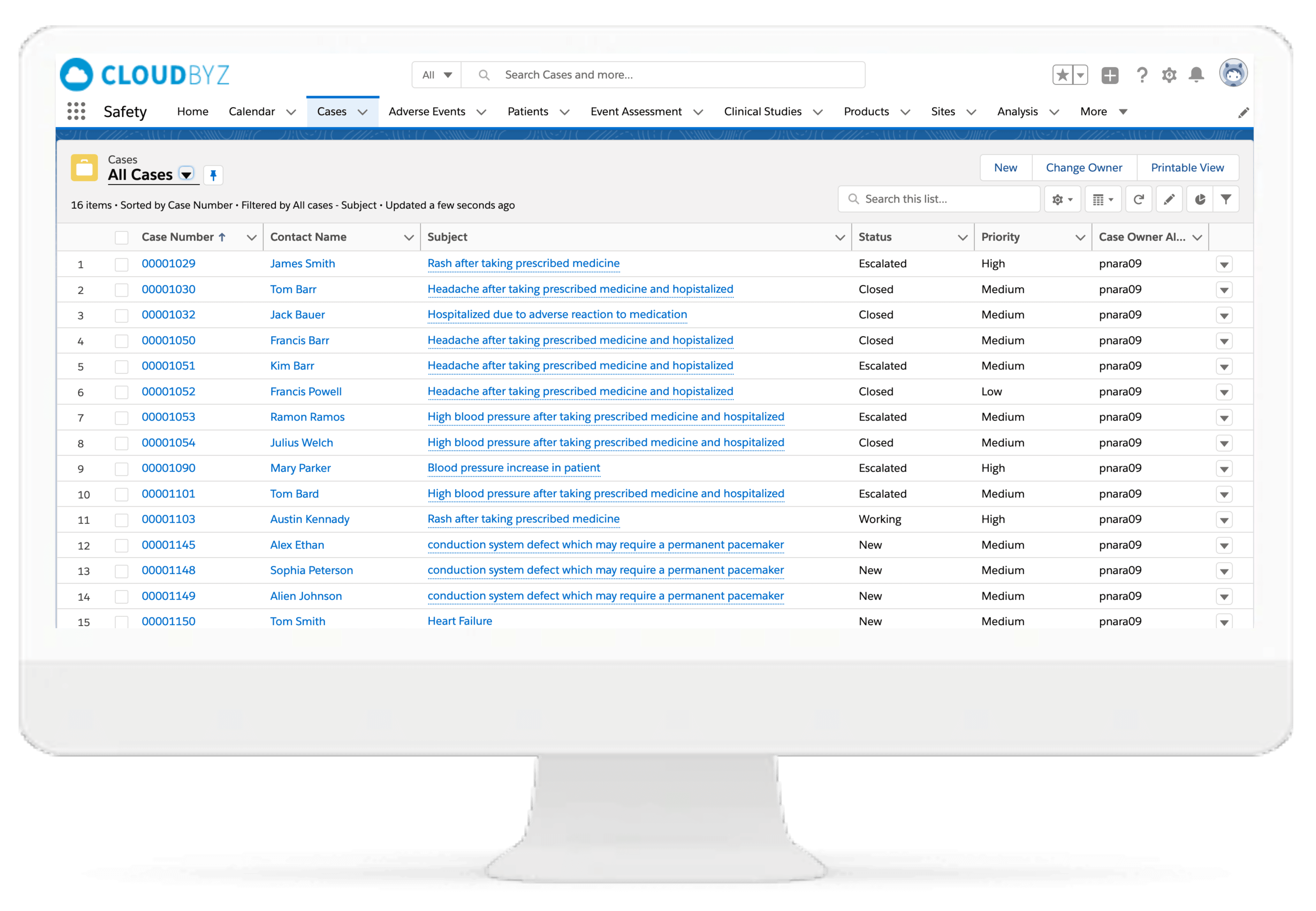
Case Management
Cloudbyz pharmacovigilance software solution enables resilient processes. It ensures scalability and end-to-end global case processing and safety process, E2B intake and submission, periodic reporting, etc.
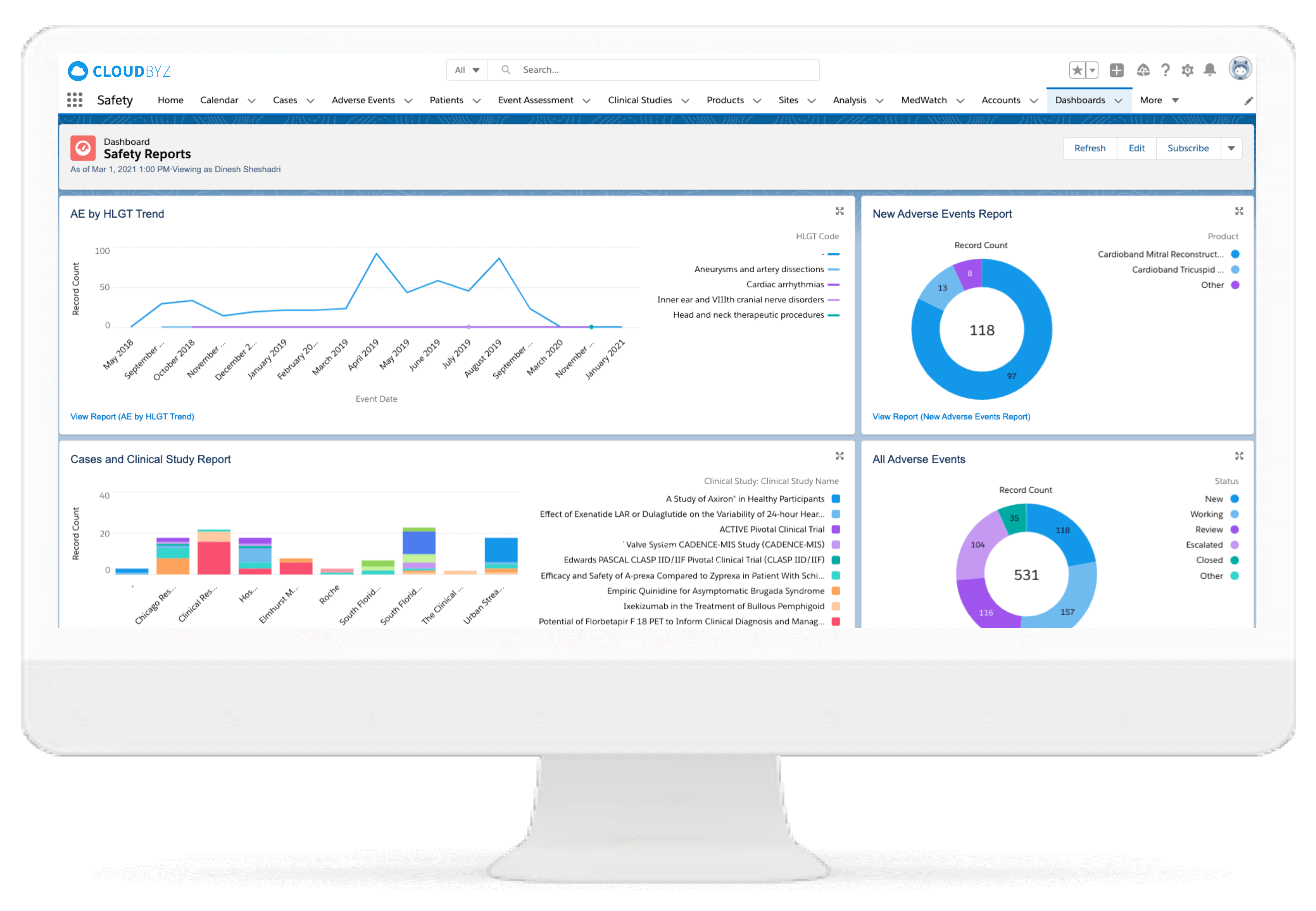
Adverse Event Management
The adverse event management ensures collaborative and detailed adverse events reporting with PSUR, PADER, ASR, DSUR, etc. This helps in faster routine pharmacovigilance submissions with better accuracy.
Patient Management
Our Safety & PV solution supports flexible report data tracking and management. It also ensures secure and centralized storage of patient information and lab test results.
Regulatory Reports
Our solution enables regulatory report generation based on pharmacovigilance rules and is configured for report seriousness and causality. It also offers a centralized view of all scheduled reports.
Service Cloud Extension
The service cloud extension functionality supports safety and pharmacovigilance through functions of the Salesforce cloud platform with extension of service cloud for medical, pharma and biotech firms.
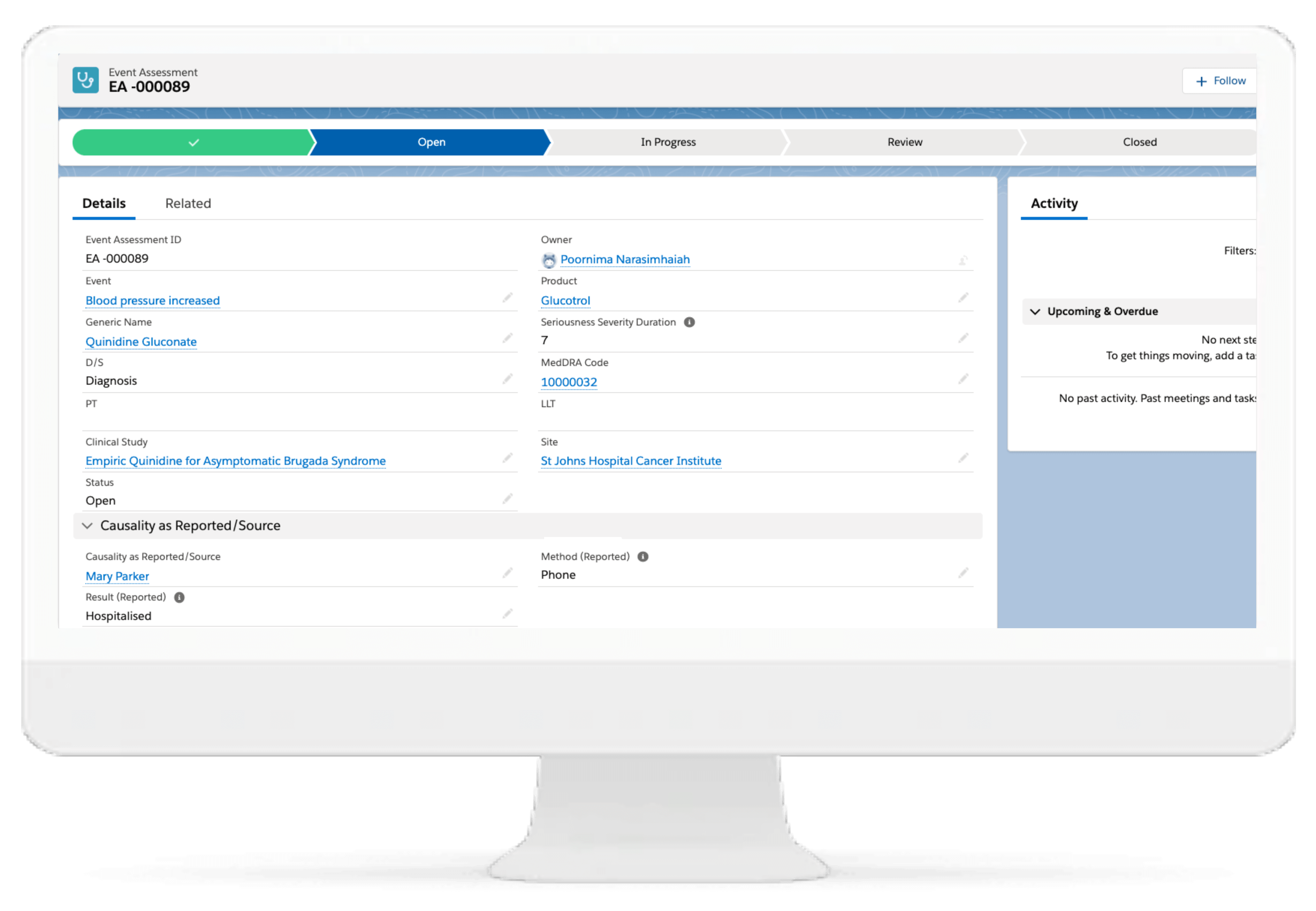
Event Analysis
Event Analysis functionality enables generation and review of narrative case description. Supports additional data entry for generating the MedWatch 3500A and BfArM pharmacovigilance reports.
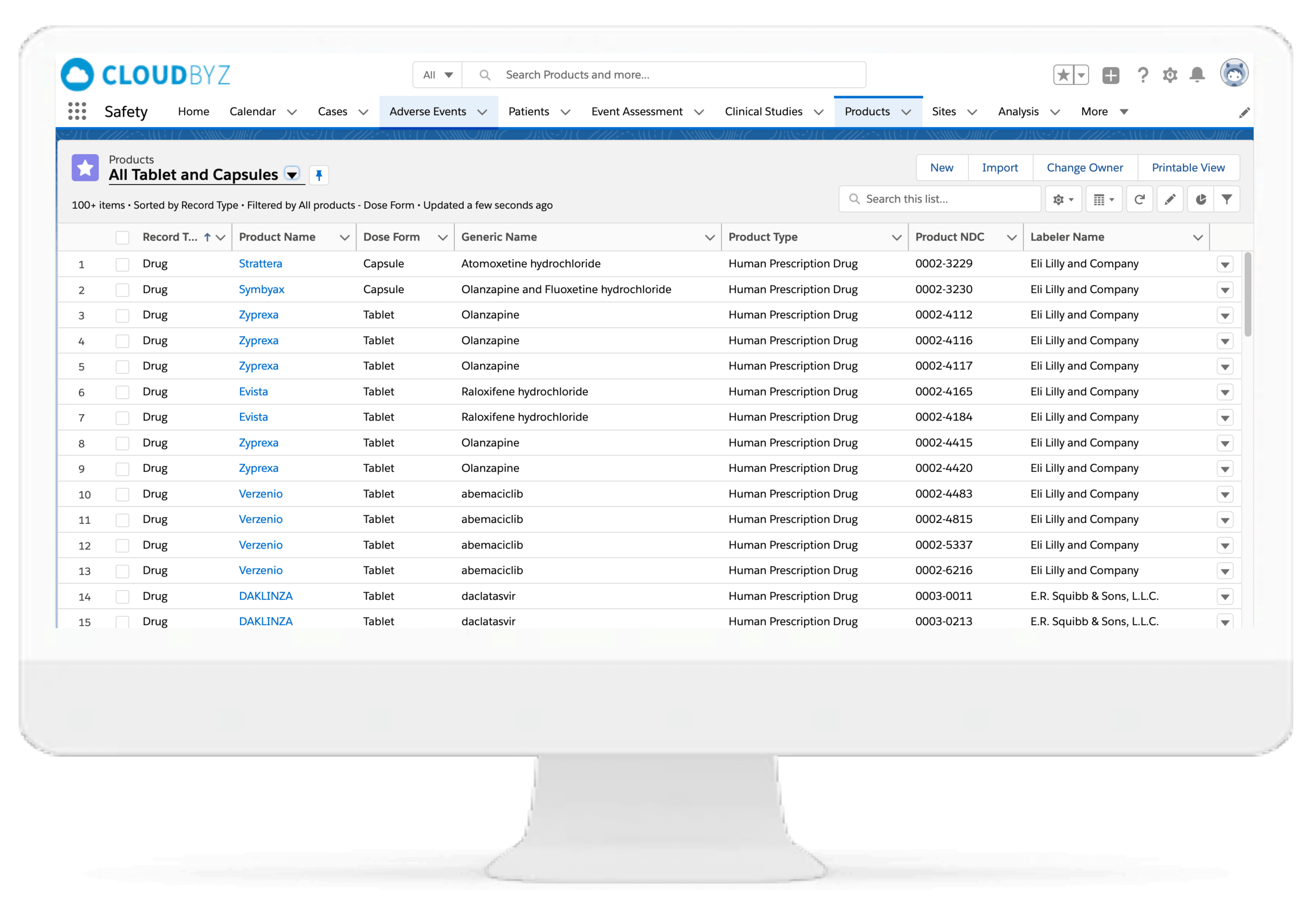
Product Management
This functionality can improve pharmacovigilance (PV) by recording and managing details regarding products and dosage regimens while building associations for pharmacovigilance data accuracy.
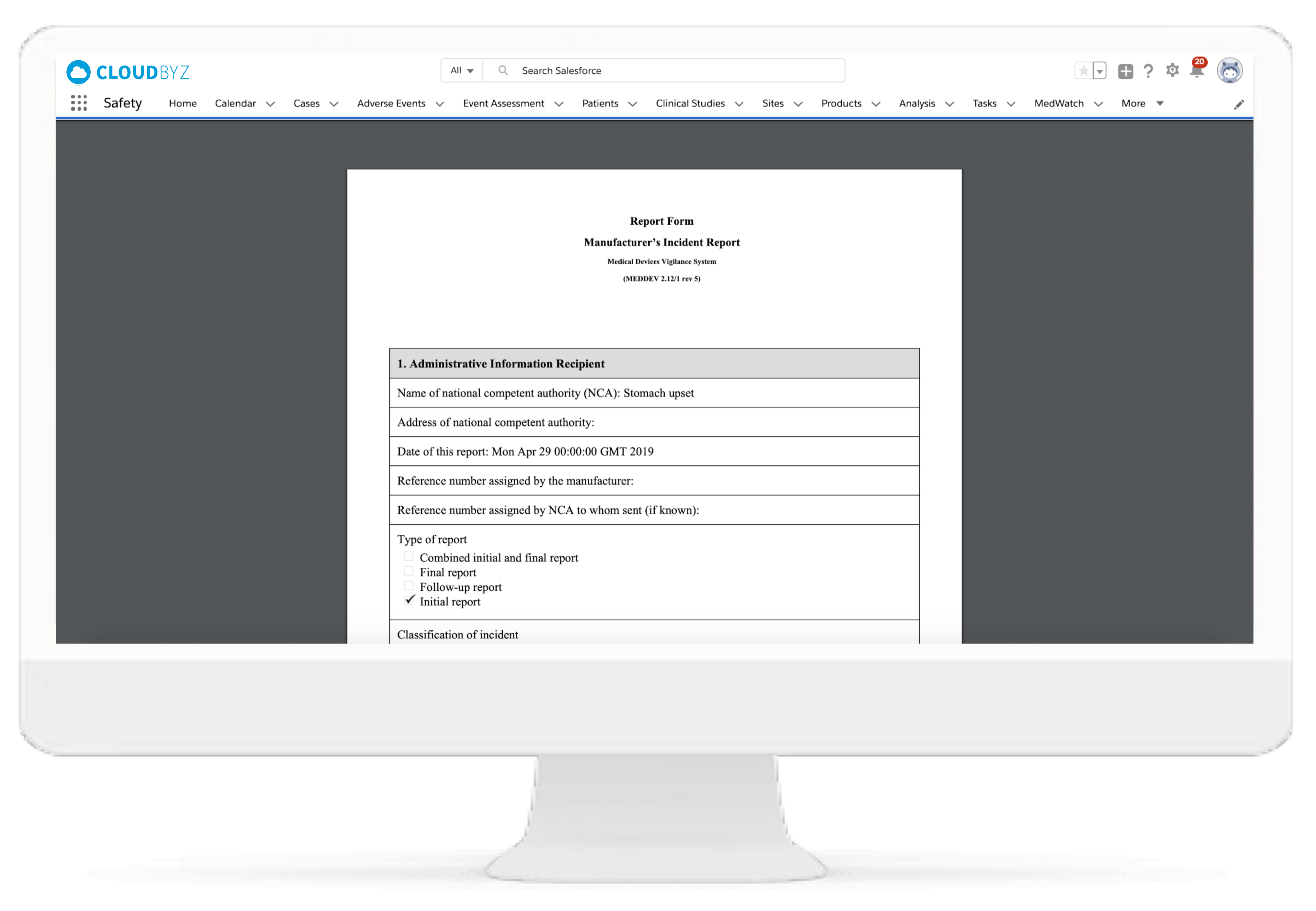
Medwatch Information
Cloudbyz pharmacovigilance platform enables easy additional information entry in the MedWatch 3500A report. This is used with case and adverse event details to generate regulatory and MedWatch reports.