Product Overview
CTMS (Clinical Trial Management System)
CRA Activities Monitoring. Subject Tracking & Invoicing. Investigators & Sites Management.
Automate your routine CRA tasks with CRA Activity Management
Create confirmation and follow-up letters in one click with all flexible smart trackers added automatically.
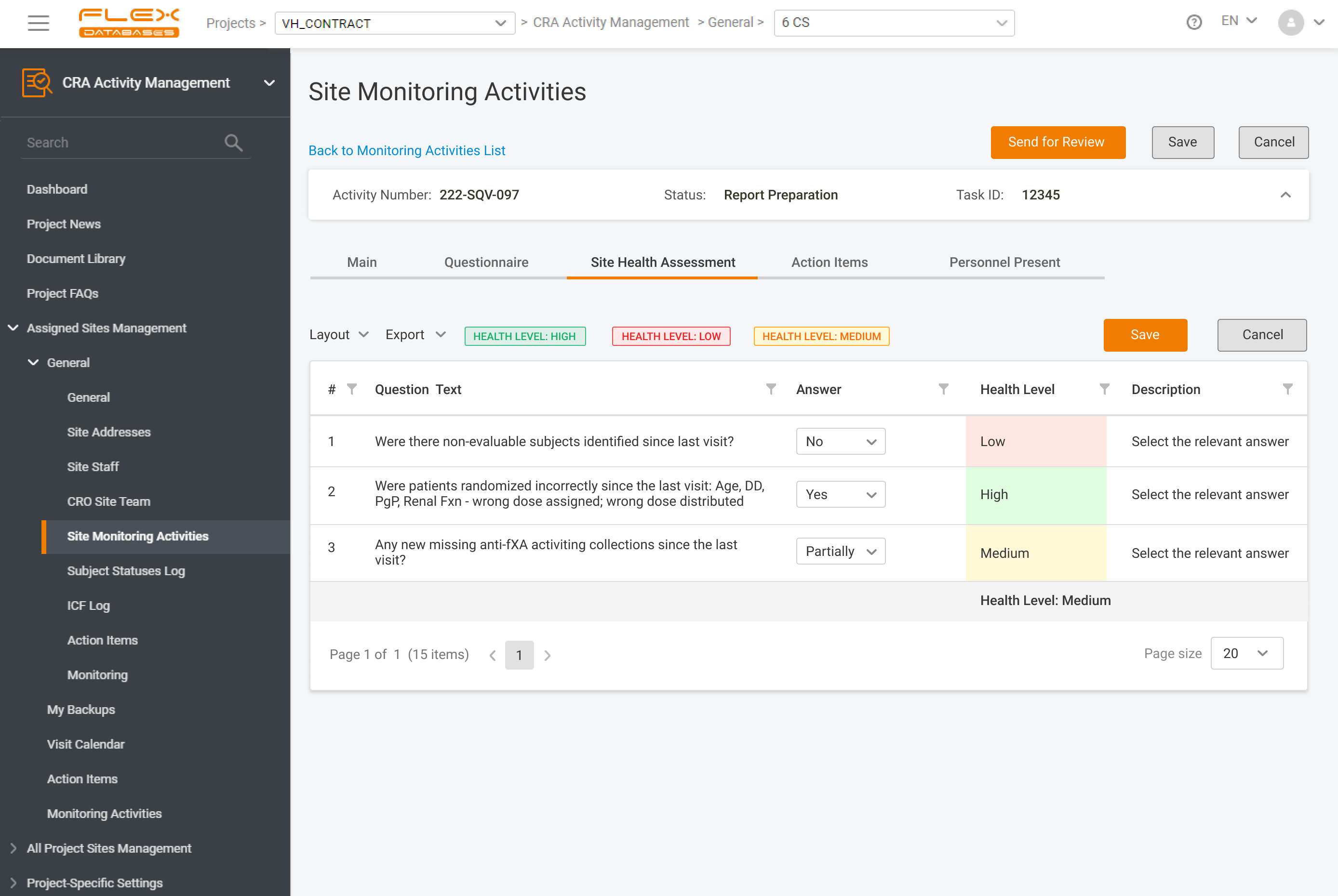
Configure Site Visit Report workflow with electronic signatures to replicate your exact processes.
Poor internet connection at site? Use offline reporting capabilities of the system.
Track what is going on at sites with Subject Tracking & Invoicing
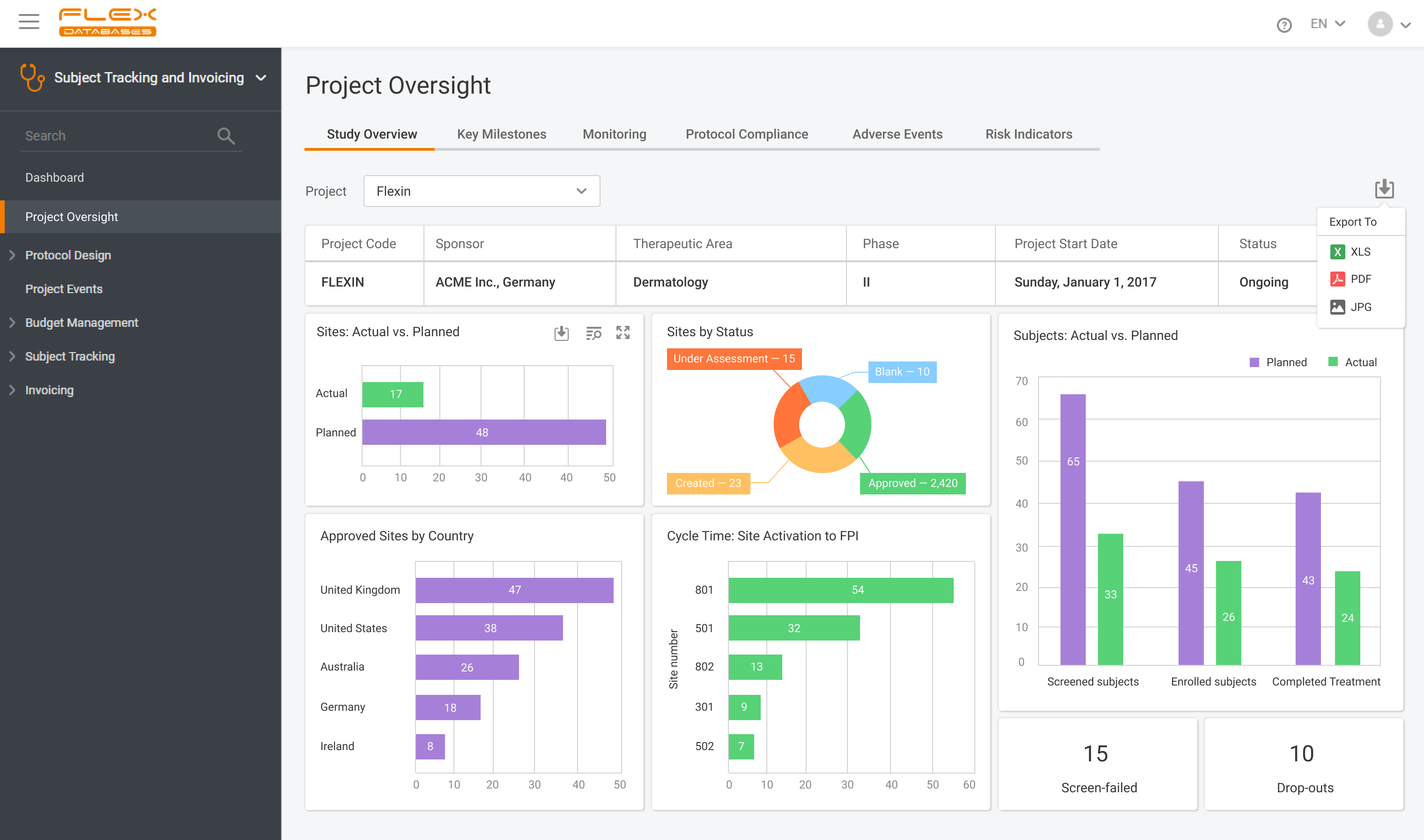
Have all the information you need in one place: enrollment, inclusion curve, in and out of time window visits, screen failures and so much more.
Use flexible and exportable widgets, reports, and graphs.
Set, measure, organize, and track site payments.
Organize all information on investigators and sites with Investigators & Sites Management
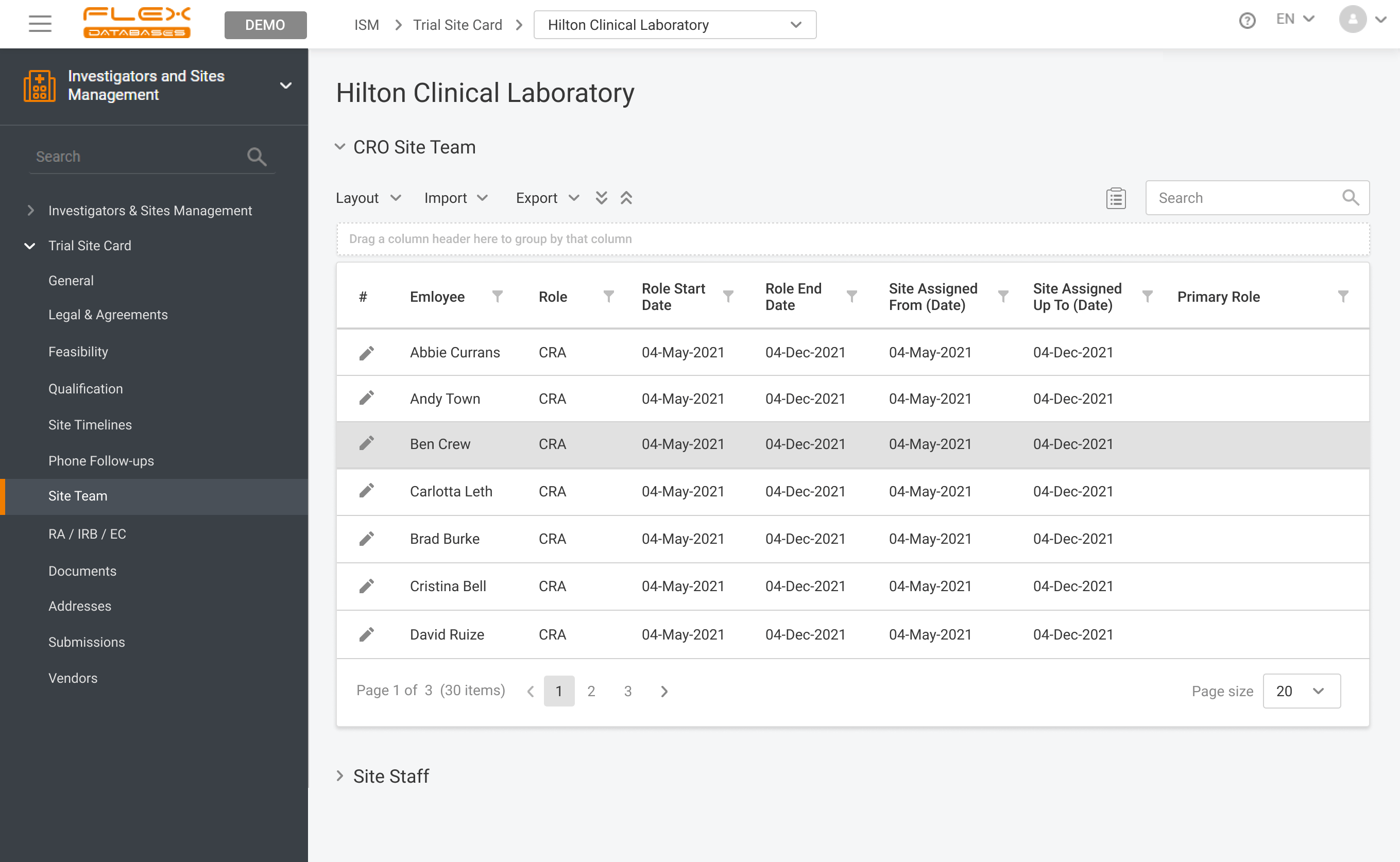
Have sites statuses, documents, contracts, qualification, vendors, timelines and so much more at hand.
Keep all your Medical Institutions and Sites information in a clear organized manner with just a few clicks.
Manage submission packages across countries, studies, and sites in a unified interface.
Specifications
CRA Activity Management
Site visit planning and scheduling: Pre-Study, Site Initiation, Interim Monitoring, Monitoring Visits, Close-Out, and any other type
Study role-based permissions
Automated notifications and alerts based on various parameters
Assessment metrics creation and CRA performance assessment
Business Intellig ence reporting – ANY report is possible
Customizable fields & trackers – all fields in monitoring section can be changed by user at no extra fee
Offline Site Visit Reports – you no longer depend on internet connection at sites
API for integrations – full integration with TMF, EDC systems
Sponsor step: enable optional sponsor step in the workflow to allow sponsor review and sign reports
Centralized calendar and personalized calendars of planned visits
Electronic signature
Monitoring Visits reports, confirmation & follow-up letters generated automatically on customers templates
All trackers are completely flexible and configurable – action items, issues, deviations, subjects enrollment and any her logs
Templates designer for all documents – full flexibility with confirmation letters, follow-up letters, site visits reports d questionnaires
Site visits workflows are flexible and can be configured to reflect customer’s exact process
Ad-hoc reporting tool for cross-project and cross-module reporting – graphs, widgets, pies, grids – all exportable
Subject Tracking & Invoicing
Create study and multi-currency site budgets
Set up any payment rules with overhead percentage, cost and extra cost reductions
Copy budget template to speed up trial set-up
Track non-visit related activities (PTC management)
Manage open queries resolution
Plan and schedule patients visits
Import data from your EDC system or add subjects manually
Generate invoices & beneficiaries on configurable client-specific or ready- to-use templates
Void or approve invoices online
Invoice based on various triggers: visit, procedures, milestones, etc.
Keep different site budget versions and invoice according to specific version
Export and report any data on invoicing into sites
Track unscheduled visits and procedures
Plan enrollment and compare with the real picture
Track all patient related data
Get overall reports on ready to be invoiced, approved and paid activities
Track invoices status
Investigators & Sites Management
Feasibility process
Site performance
Centralized IRB/LEC submissions and approval tracking
Investigators, sites, hospitals and out-patient clinics, vendor and regulatory authorities information
Site team assignment
Capturing and tracking of all site communication
Documents tracking (contracts, site regulatory documents, licenses and certificates)
Reports