Product Overview
Flex EDC
Flex EDC – ensure fast capturing and reviewing data of your clinical studies in a user-friendly interface
Create, manage and control your eCRFs
Have a complete track of your study at one place
Speed-up your study start-up timeline – only 3 weeks to set up FlexEDC for your trial
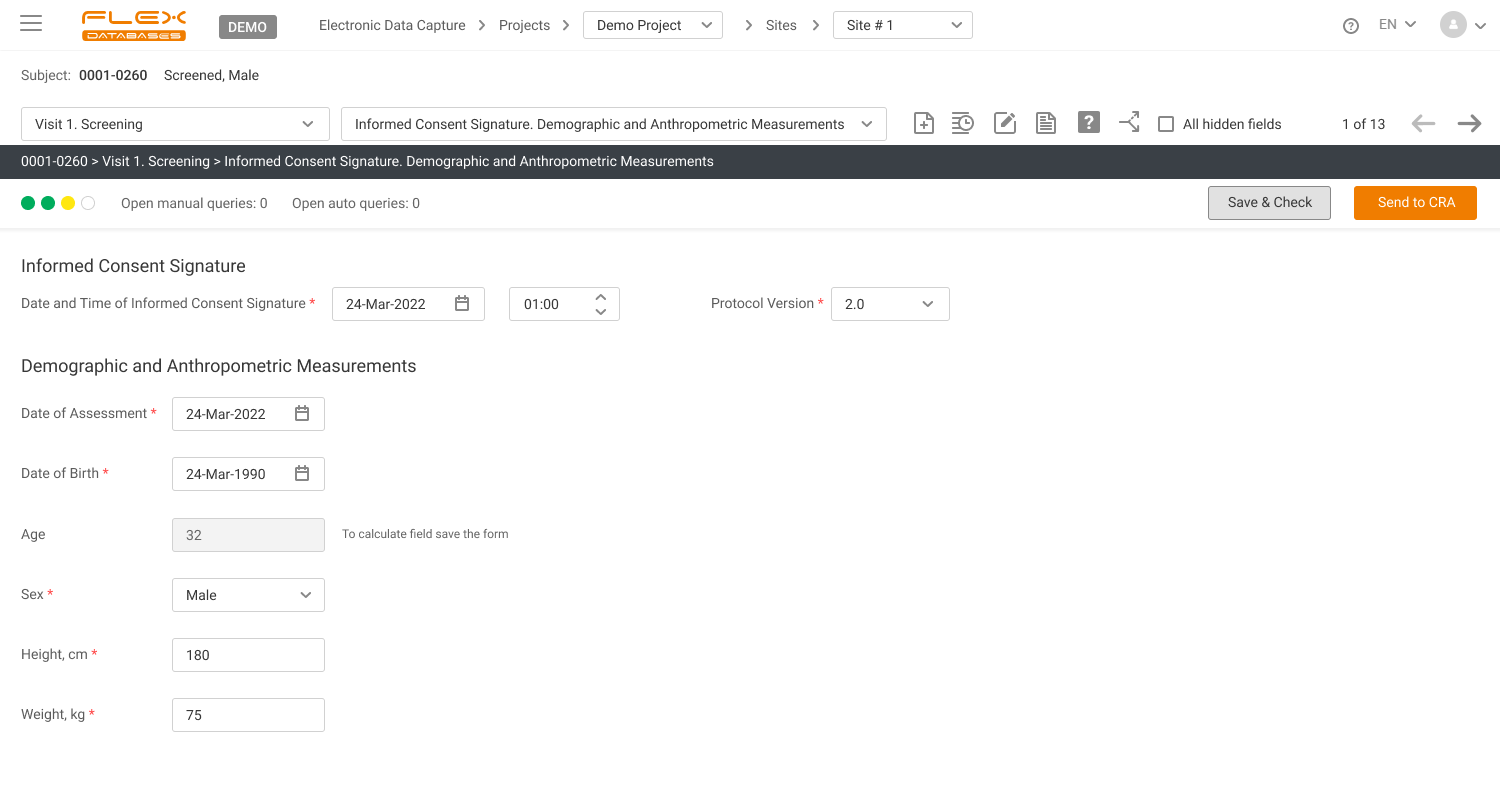
Arrange fast and easy completion and check of your eCRFs
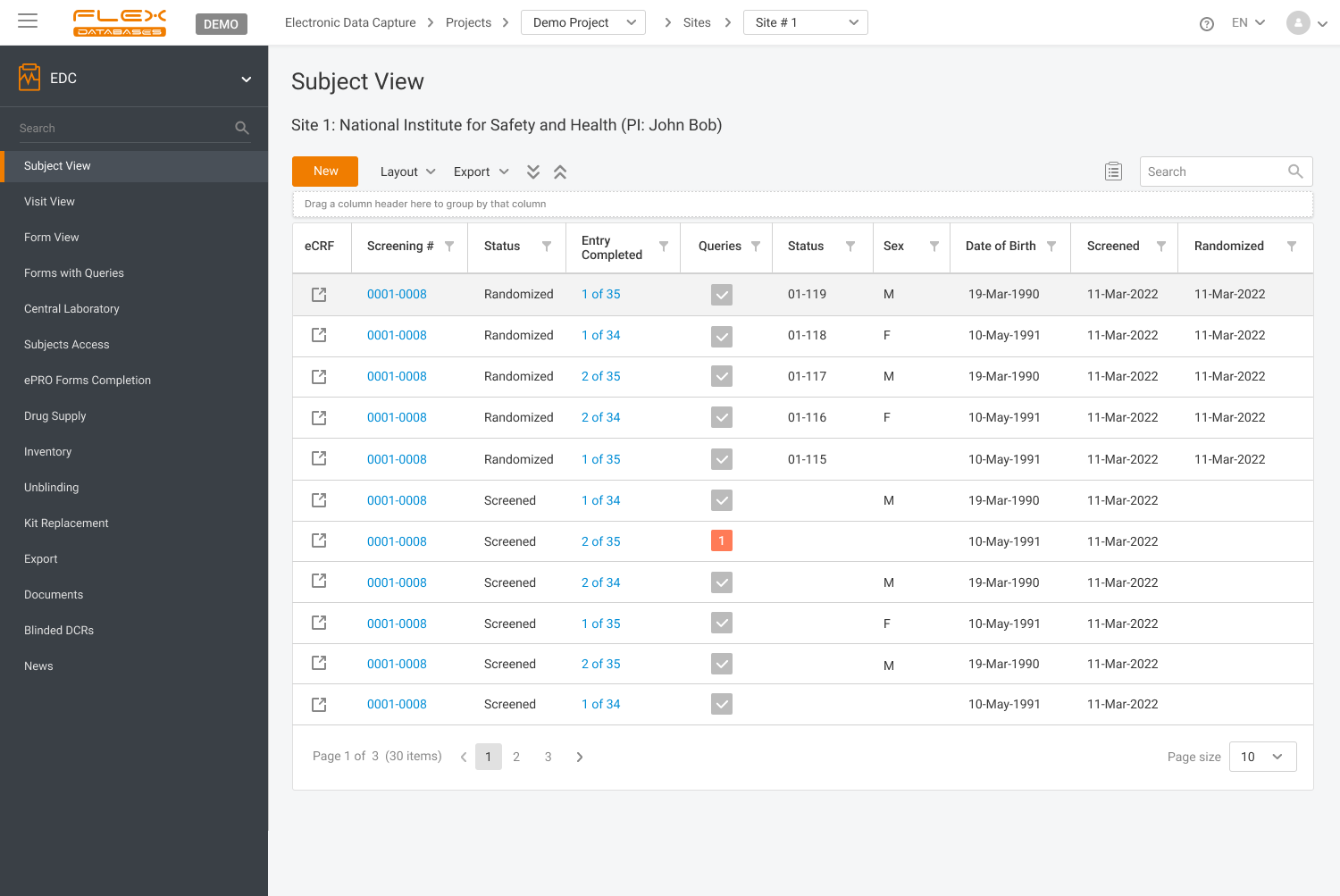
Control all the visits and eCRF fulfillment in one screen – a convenient and straightforward semaphore system enables to have a glance at all patients' visits and eCRFs completion
Use a fully validated and 21 CRF part 11 compliant system – Flex EDC is developed in compliance with all the requirements: GCP E6 R2, 21 CRF part 11, GAMP5, HIPAA, etc.
Sprint through building your eCRFs
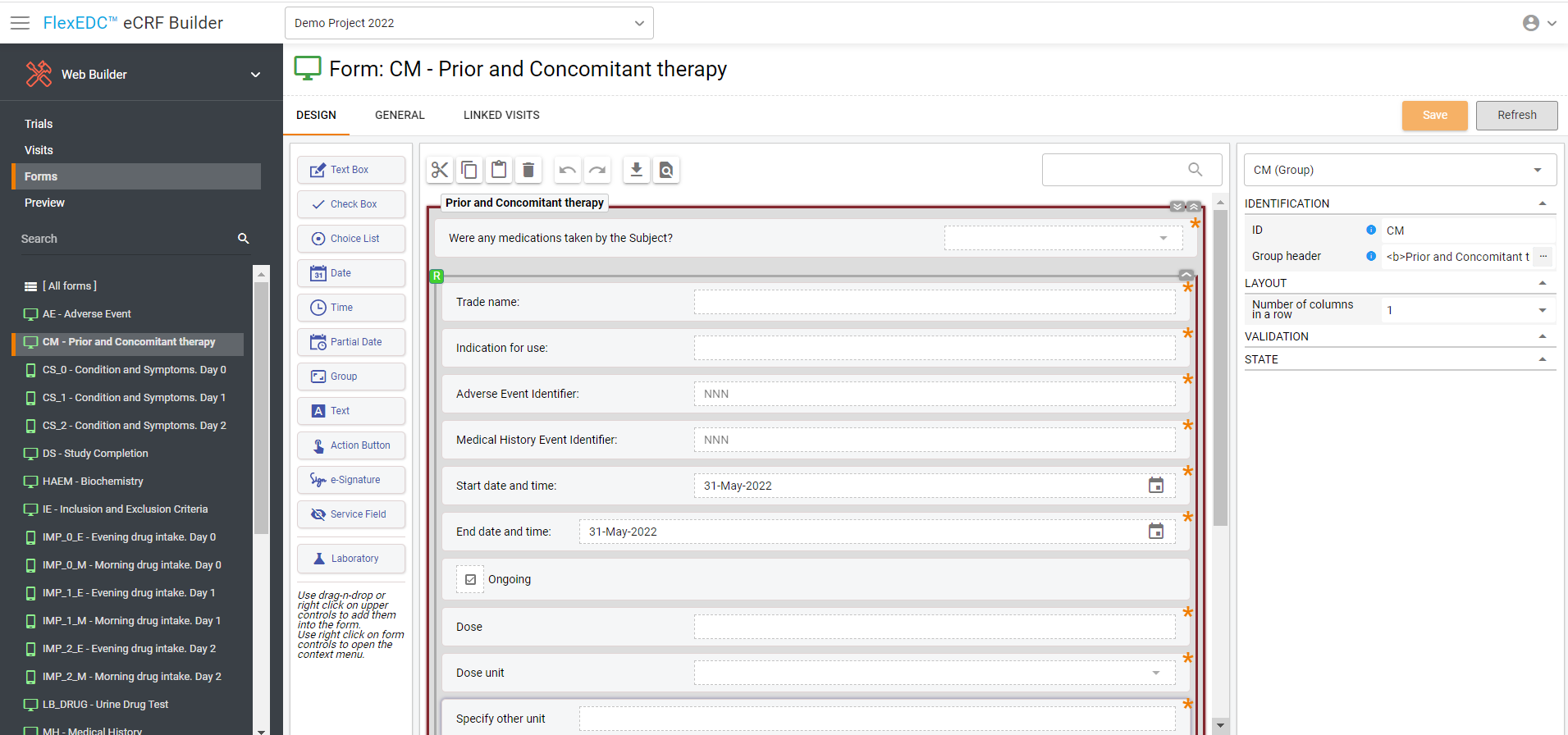
An intuitive eCRF builder enables to create forms in a few minutes
Drag-and-drop approach for a simple eCRF building
All the fields you might need are already in the system
Online set-up of calculations and dates
Manage your stock and patient randomization with IWRS
Save your time and money – IWRS is already a part of our Flex EDC: you will not be charged for it separately and always have access to the module
Use one of 4 randomization types in accordance with your study design
Randomize your patients by one click
Control your stock and IMP distribution
Supply your patients with our ePRO
Increase patient recruitment by simplifying patient’s diaries fulfilment
Have access to patient diaries in real-life time
Prevent patient dropout by managing their outcomes in real time
Minimize possible mistakes of sites and investigators
Provide your sites and investigators with a separate system and an immediate data flow to your major platform
Create mass queries in Flex EDC and use any templates you need
Arrange automatic fulfilment of the fields with possibly calculated data, excluding any potential mistakes
Let your investigators control visits with a calendar for every subject
Specifications
Simplifying process for Sponsor
Unlimited clinical trial size – up to 500 000 subjects included in one study during beta testing
Prompt and easy eCRF creation
Distribution of roles in just one click
Build-in medical coding WHODrug, MedDRA, ATC, and ICD
Export to CDISC SDTM, SAS, XML, and Module
Smart search within EDC
Semaphore system to quick assessment of processes completion
Templates for raising queries
Compliance with GCP E6 R2, 21 CRF part 11, GAMP5, HIPAA
Full-trial KPI report
Simplifying process for Investigators
Fast access to the system through a dedicated portal for Investigators
Quick and clear data input
Easy to add subjects in the study
Calendar of visits for every subject and the trial in general
Templates to close queries in a few seconds
Simple navigation system with ability to find a subject in one click
Simplifying process for patients
ePRO App for patients to simplify diary maintenance
User-friendly & supportive interface and diary management process
Branding for mobile apps & website
Fast access to patient’s diary
Integration with wearable devices, such as fitness trackers and smart watches