Product Overview
Pharmacovigilance
Flex Databases Pharmacovigilance is a comprehensive drug safety database that ensures robust and compliant pharmacovigilance activities both within clinical trials and post-approval stage. End-to-end process of collecting, triage, evaluation and submission of safety data in a single point.
Full-cycle pharmacovigilance
Create or align your own pharmacovigilance process with the system by utilizing the embedded, fully configurable workflow
Communicate with partners and regulatory authorities directly via EDI gateways
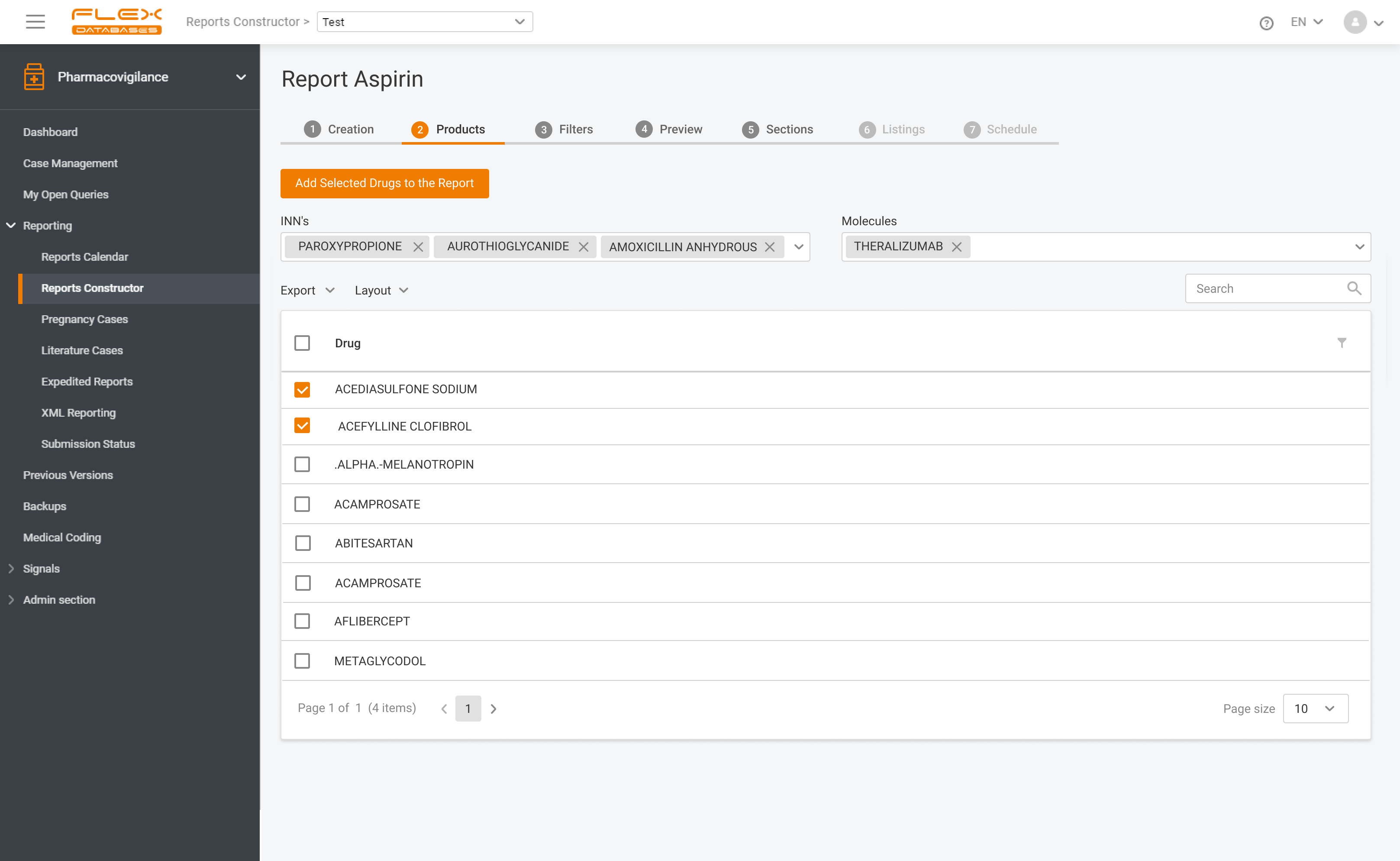
Make reports in any format, required by regulatory authorities
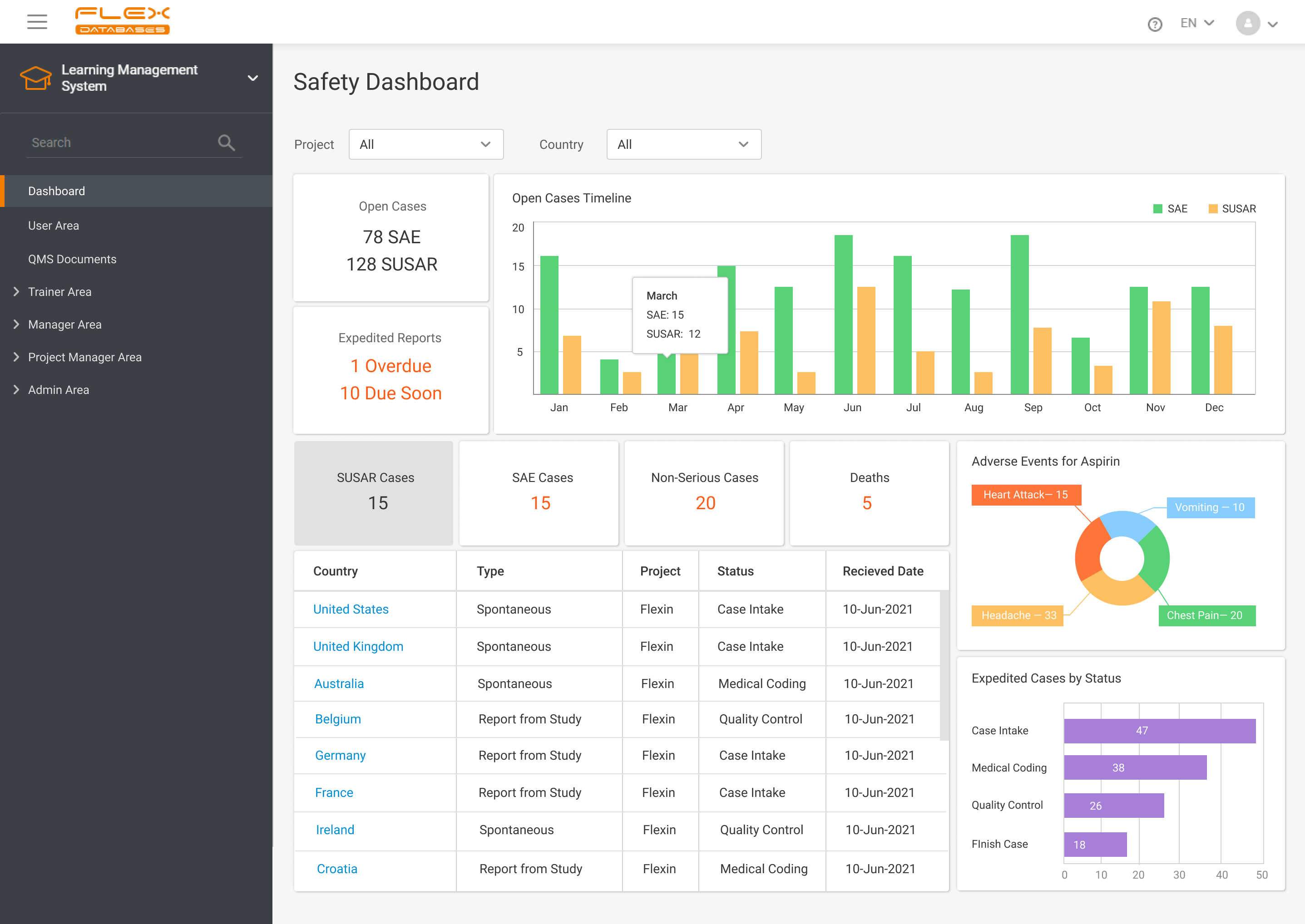
Data-informed decisions on a drug safety profile
We leverage the latest technologies for advanced safety data analysis and evaluation
Our reporting tool kit is designed to provide instant data accessibility for further interpretation and actions
Qualitative and quantitative (statistical, machine learning, neural network) methods for signal detection and management
Specifications
General
Multi-language input
Configurable workflows
Multi-tenant access
Data export in Excel, Word, XML and PDF
In-built MedDRA coding tool with auto-coding option
Comprehensive Audit Trail
Cross-field validation to ensure E2B compliance
Query chat for communication
Periodic submissions
PSUR/PBRER/DSUR line listings
Summary tabulations builder
Reports calendar to manage periodic reports timelines
Paper-based and electronic reports
CIOMS I
MedWatch 3500A
E2B R3 compliant XML report
Local reporting forms on-demand
Follow up reports management
Advanced analytics
Signal detection and management using qualitative and quantitative (statistical, machine learning, neuralnetwork) methods
Ad-hoc reporting tool
Duplicates search
Data reconciliation with third-parties
Integration with EDC / Clinical databases
EDI Gateways with partners or competent authorities
API-based data export
E2B R3 XML import
Reporting Timelines
Submissions tracker with country-specific setup of reporting timelines
Notifications / alerts on actions to be taken and upcoming deadlines Download RFI