Product Overview
Clinical Trial Management System (CTMS)
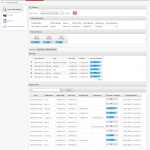
Octalsoft CTMS is a cloud-based clinical trial management system that transforms your disjointed back office clinical trial tasks into a highly efficient and cohesive work environment. It enables global pharma sponsors/CROs to maintain a centralized, relevant and most up to date study and operational database; thus providing users with real-time operational visibility and total control. The system complies with all industry best practices and regulations in streamlining and automating the trial processes in a compliant manner. It allows you to map out the entire clinical trial lifecycle, right from recruiting to reporting, so that your research team can easily keep track of activities they need to perform, in the order, they need to perform.
Why choose Octalsoft CTMS?
The CTMS software solution from Octalsoft is a collection of eTools that are guaranteed to change the way life science organizations, pharmaceutical, and medical device companies conduct and control clinical trials. Right from site identification to study closeout, the tools empower you with various capabilities to manage all your critical processes with ease and flair.
Equipped with a live digital dashboard, the system helps you manage calendars, milestones, deadlines, documents, risk-based monitoring, subjects and financials, all in a scalable feature set. It aims to improve efficiencies in planning, resource management, compliances, risk mitigation, data quality, and work processes while reducing operational expenses and accelerating the trial life cycle.
Specifications
Centralized Database
Octalsoft CTMS regularly syncs and aggregates all trial data into a consolidated, centralized database. A shared source of real-time integrated data facilitates collaboration amongst study team members and favors proactive management. This allows one to make time-critical decisions and resolve issues promptly.
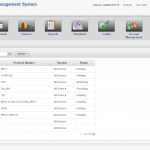
Clinical Site and Subject Management
Octalsoft CTMS allows users to track study progress from start-up through enrollment and progression throughout the study timeframe. The integrated subject calendar helps track subject visits and milestones. User can define metrics for each study utilizing study set-up templates and study creation wizards.
Risk-based Monitoring
Centralized monitoring tools and data analytics, enables the user to identify, evaluate, and mitigate the risks that could affect the quality or safety of a study. User can define thresholds for pre-identified risks and visualize these through live dashboards or generate periodic reports.
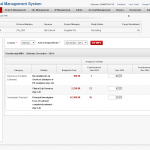
Finance Management
Octalsoft CTMS, helps you manage your trial budget and site contracting terms efficiently. It enables improved control over the process by integrating required workflow in invoice generation, partial payments and payment histories. The system generates investigator pay-outs automatically based on pre-agreed milestones.
Quality and Compliance management
For an industry so heavily regulated, Octalsoft’s CTMS quality control and compliance management module helps you make sure that your processes adhere to industry regulations and your study team is always compliant and audit ready.
Collaboration
A single source of real-time database facilitates collaboration amongst global study team members and favors proactive management. This feature is further enhanced by automated notifications and escalations for quick actions. The system also maintains proper controlled access and security checks appropriate to given user/team.