Product Overview
Electronic Data Capture (EDC) Software
Octalsoft Electronic Data Capture software is designed to streamline the collection of trial patient data, that supports early to late-stage clinical development phases and post-approval trials. The flexible and scalable EDC solution finds patronage across the complete spectrum of clinical research community including Pharma, Biopharma, Medical Device Companies, CROs, and Clinical Sites. Octalsoft EDC is a comprehensive cloud-based software with a responsive layout that provides a simple user interface to capture trial subject data with ease. With integrated online edit checks and query management features, users can capture quality data with speed and accuracy. The system’s powerful reporting capabilities help study teams visualize and analyze study data in real-time. By leveraging open standards, it allows for easy integration of clinical and non-clinical data from multiple sources. In a nutshell, Octalsoft EDC accelerates the speed of your clinical trial by reducing deployment time, capturing clean data quicker, timely study close-out and early data lock – all in a cost-effective manner.
Why choose Octalsoft EDC?
Because it’s simple, fast and affordable and gives a frustration-free data capture experience!
Designed with User Experience (UX) always at the forefront
Smart study designer to set up the most complex studies with flair
Captures high-quality data over a secured network
21 CFR Part 11 compliant
Adheres to HIPPA privacy rules
Multi-platform and multi-device enabled
Comes with an integrated medical dictionary (MedDRA, WHO Drug, and More)
CDISC compliant
24×7 support – Our experienced support team is always ready to help, so you never walk the path alone.
Specifications
Intuitive User Interface (UI)
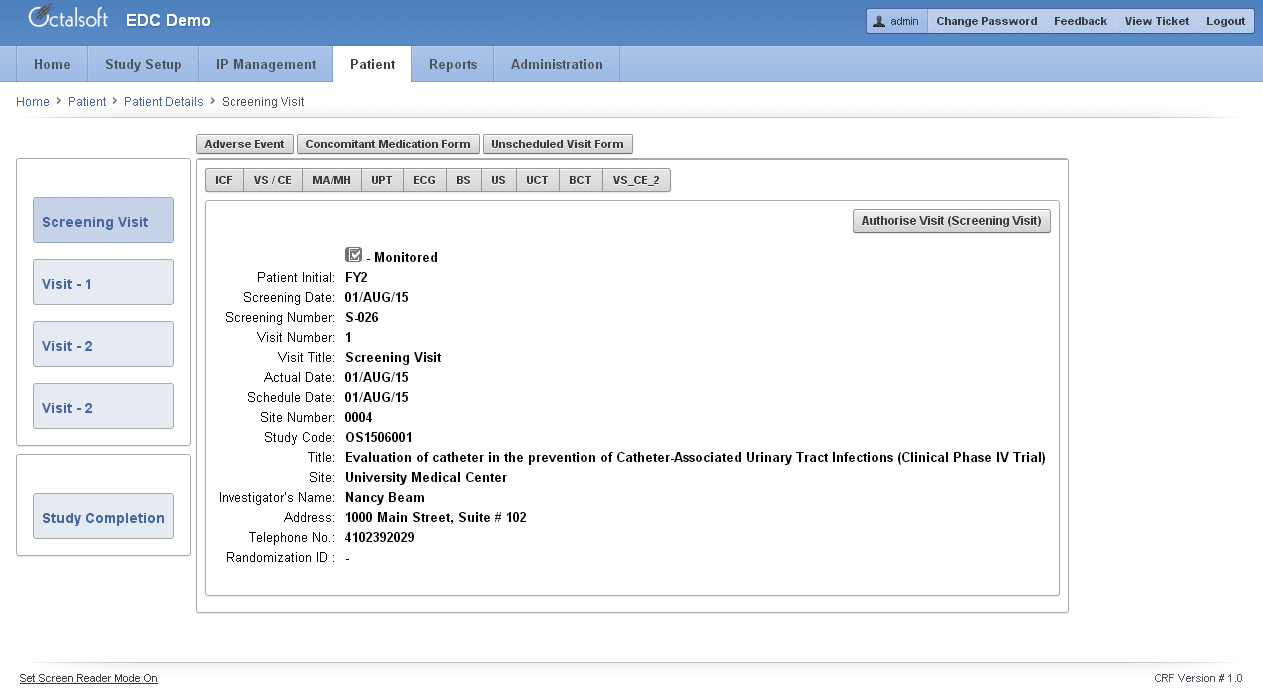
A confusing UI can dilute the efficiencies of an EDC system. Octalsoft EDC’s UI, with easy navigation, is intuitive enough even for new users to get the hang of it quickly. Simple page layouts, dynamic questions, chronological response mechanisms, responsive autocomplete fields, conspicuous progress bars, minimal free text entries, unambiguous error messages – all make the difference between valid, timely data entries versus no entry at all.
Study Planner
Robust study planner with unlimited data fields enables setting up complex calculations and field dependencies in a matter of weeks. It allows cloning and reuse of study components across trials and simplifies study configuration.
eCRF Designer
Octalsoft EDC’s friendly eCRF form builder allows you to efficiently build and manage multiple eCRFs across various trial phases. The system helps you design your eCRFs with a simple drag-and-drop interface, without the need of any IT support. You can also make mid-study eCRF changes without any data migration requirement or taking your study offline. The system further allows control over eCRF visibility by assigning user-specified roles.
Online Data Validation
Real-time edit checks at the point of data entry ensure that the data generated is clean and accurate. The system re-orients the user when their input conflicts with pre-defined field requirements. Various system-level checks quickly identify transcription errors, missing and duplicate data. Skip logic eliminates irrelevant fields from user view – for example, why show entry for pegs per day if the patient is non-alcoholic?
Query Management
Streamlined communication between monitors, data managers, and study coordinators ensure issues with data discrepancies are addressed promptly. Our system not only comes with the capability to manually add queries but also auto-generate queries based on logic validation. Integrated alerts and reminders ensure measures are taken consistently. The system further tracks and records, if all queries are responded to and resolved by defined users before data is locked.